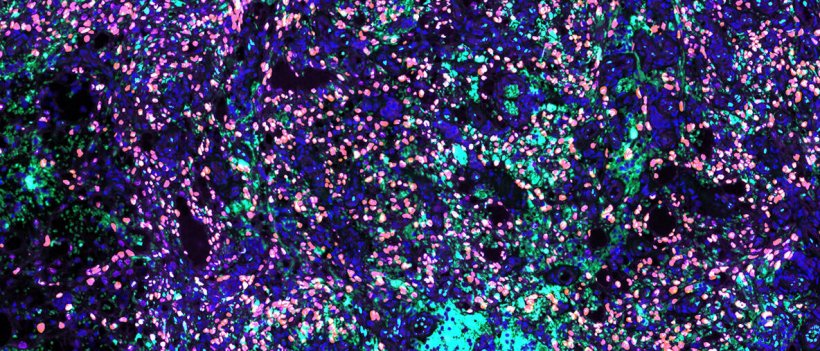
Photo: Ojala lab/University of Helsinki
News • Viral oncology
Kaposi Sarcoma: Answers to a longstanding enigma
The oncogenic herpesvirus (HHV8 or KSHV) causes a cancer known as Kaposi’s Sarcoma. An international team of scientists led by the University of Helsinki has discovered key factors that control the genome maintenance and replication of a virus responsible for lymphatic vascular cancer.
Kaposi Sarcoma (KS) is the most common cancer among AIDS patients and it is often seen in sub Saharan and Mediterranean populations or after graft transplant. Kaposi Sarcoma is suggested to originate from the endothelium in immunocompromised individuals. This disease is caused by a herpesvirus, Kaposi Sarcoma herpesvirus (KSHV). KSHV displays two modes of infection, latency and lytic phase, with both phases contributing to the tumorigenesis. Latency represents the default mode of infection in virtually all susceptible cell types. Quite exceptionally, lymphatic endothelial cells (LEC), the suggested KS tumor cells precursors, display a unique viral infection program with spontaneous lytic gene expression as well as high intra- and extracellular viral load.
In the current publication in Cancer Research, a journal of the American Association for Cancer Research, a collaborative team of researchers led by Professor Päivi Ojala decided to address a long-standing question: What is the molecular etiology of the spontaneous lytic phase observed in KSHV-infected primary human lymphatic endothelial cells (KLECs)? “The research group discovered that two central proteins that directly regulate gene expression on the DNA are key factors, control the genome maintenance and replication of a virus responsible for lymphatic vascular cancer. These two factors support the virus-induced tumor formation and represent new attractive therapeutic targets,” Ojala says.
Silvia Gramolelli, an Academy of Finland post-doctoral researcher at the University of Helsinki, investigated the role in Kaposi Sarcoma of PROX1, SOX18 and COUPTF2, the three key transcription factors (TFs) governing the birth of the lymphatic vasculature during embryonic development. The results of the study demonstrate that all three key factors are expressed in a cohort of KS tumors concomitant with unexpectedly prominent marker of the late lytic phase, the protein K8.1. “Key transcription factors SOX18 and PROX1 expressions are crucial to support the spontaneous and productive KSHV lytic infection program in LECs, and their expression in tumors correlates with lytic and latent markers of infection,” says Gramolelli.
This study uncovers how the virus mechanistically hijacks PROX1 and SOX18 to its genome to support its own replication and lytic gene expression
Silvia Gramolelli
This finding turns around the current dogma that regards the KS tumor cells predominantly latently infected and indicates that lytic gene expression is far more abundant than previously thought. “To our surprise, SOX18 and PROX1 regulate two different and complementary processes in the virus oncogenic replication cycle independently from each other,” says Gramolelli. “While PROX1 enhances the viral lytic gene expression, SOX18 is involved in the maintenance of a higher number of viral genome copies. Besides revealing an exciting insight on the KSHV infection program in endothelial cells, this study uncovers how the virus mechanistically hijacks PROX1 and SOX18 to its genome to support its own replication and lytic gene expression,” Gramolelli continues.
A companion study demonstrating PROX1-Enhanced Productive Lytic Replication of KSHV led by Professor Young Kwon Hong (Keck School of Medicine, University of Southern California) is also published as a back-to-back article in Cancer research.
Despite decades of research, no standard treatment for KS has been established. Clinical outcomes are particularly unfavorable, especially in resource-limited communities. The research group discovered in collaboration with Associate Professor Mathias Francois (the Centenary Institute, University of Sydney) that both a SOX18 small molecule inhibitor previously developed by Francois and the R-enantiomer of an FDA-approved beta blocker, propranolol, showed a striking reduction in intracellular and extracellular viral loads in vitro. “These findings suggest a potential, novel therapeutic avenue for KS, but further work is required to see if it will be possible to apply them to design new KS therapeutic strategies targeting SOX18,” Ojala says.
Source: University of Helsinki
09.06.2020