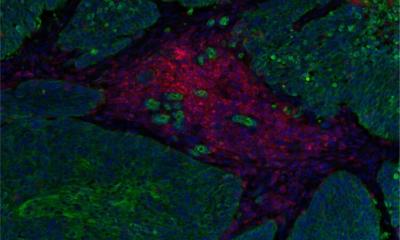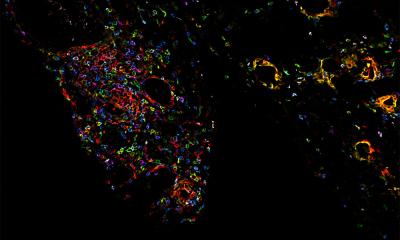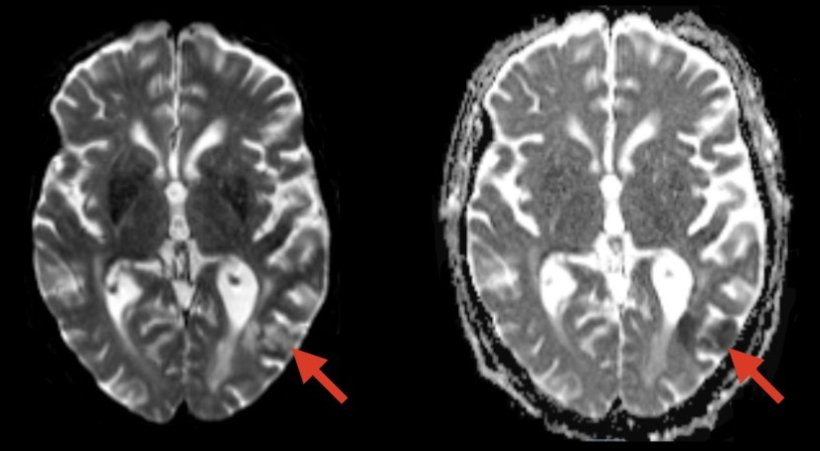
© Dr Omar H. Butt of Washington University at St. Louis
Article • Cancer patients at risk
Blood test detects risk of neurotoxicity from CAR T-cell therapy
Chimeric antigen receptor (CAR) T-cell therapy is an immunotherapy treatment that re-engineers a patient’s own T-cells to help them attack malignant tumour cells. It has been very effective in the treatment of blood cancers, including certain types of leukaemia and lymphoma. However, two serious side effects are common as a result of the treatment: cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS).
Report: Cynthia E. Keen
Researchers in Germany and in the United States have separately determined that high levels of serum neurofilament light chain (NfL), a protein in the blood indicative of neuroaxonal injury, are associated with ICANS complications. A simple blood test identifying NfL levels can help identify cancer patients who are at risk of developing ICANS following CAR T-cell therapy.
ICANS represents a broad spectrum of neurologic symptoms ranging from mild confusion, tremor, headaches, difficulty reading, difficulty concentrating, lethargy, and memory problems to severe brain swelling, seizures, coma, and even death. Between 40% to 60% of patients receiving CAR T-cell therapy develop ICANS.
Two independent studies, one conducted at the Washington University at St. Louis (WUSTL) and the other by a multi-institutional German team led by researchers at Ludwig Maximilian University (LMU) of Munich, have identified that higher NfL levels in cancer patients’ pre-CAR T-cell treatment correlate with the severity of subsequent ICANS.
German multi-institutional study
Principal investigator Prof Louisa von Baumgarten, MD, of LMU’s University Hospital, and co-researchers hypothesized that neuroaxonal integrity might have an important role in determining the severity of ICANS. Their hypothesis was based on numerous studies demonstrating that NfL serum levels correlate well with cerebrospinal fluid levels, mirror the extent of neuroaxonal injury, and predict outcomes for severe neurologic conditions, including multiple sclerosis, neurodegenerative disorders, traumatic brain injury, and ischemic stroke.
© Copyright 2020. St. Jude Children’s Research Hospital
Ninety-six patients being treated at CAR T-cell therapy centres at LMU and University Hospital Heidelberg had their serum NfL levels measured five days before treatment, the day of treatment, and on the day that maximum ICANS symptoms were exhibited. NfL pre-levels were significantly higher in patients who developed moderate to severe ICANS after CAR T-cell transfusion than in patients reporting no or mild ICANS. Both post-treatment and pre-treatment NfL levels correlated with the severity of the subsequent neurotoxicity.
Writing in Blood Advances, the researchers said, ‘These findings suggest that measuring the level of NfL – alone or in combination with known risk factors such as tumour burden, CAR T-cell expansion, systemic inflammation, and immune system activation – might be useful to predict severe neurotoxicity after CAR T-cell transfusion […]. Our data imply an increased risk for more severe ICANS as a result of neuroaxonal injury if NfL – pre-treatment is greater than 75 pg/ml.’
USA study
Armin Ghobadi, MD, and Beau M. Ances, MD, PhD, both of WUSTL, led a similar study, published in JAMA Oncology. They measured plasma NfL levels of 30 patients receiving CAR T therapy at WUSTL and Case Western Reserve University in Cleveland at seven time-points, starting at baseline pre-CAR T infusion to 30 days after. They analysed NfL levels in patients who developed each severity grade of ICANS and those who did not, as well as age, sex, tumour burden, history of neurologic disease, and history of neurotoxic therapies.
The risk of developing ICANS is associated with pre-existing neuroaxonal injury that was quantifiable with plasma NfL level in a subset of patients
Omar H. Butt, et al.
‘Patients who developed any grade ICANS had significant elevations in baseline NfL level,’ they write. ‘Baseline NfL level correlated with ICANS grade. No association was observed with demographic, oncologic, neurologic, or exposure to neurotoxic risk factors, such as CRS. The risk of developing ICANS is associated with pre-existing neuroaxonal injury that was quantifiable with plasma NfL level in a subset of patients.’
WUSTL lead author Omar H. Butt, MD, PhD, tells Healthcare in Europe that the team is scaling up to wide scale testing in the near future. ‘We are also trying to determine where in the brain this injury originates, how this injury interacts with the immune system to develop symptoms, and how long the injury lasts after symptoms along with its lingering consequences. All these questions are under active investigation with an ongoing prospective trial integrating advanced neuroimaging with blood and spinal fluid biomarkers and neuro-physiological testing in patients undergoing cellular therapy.’
15.06.2023