The coming era of genetic medicine
By Stefan G Ruehm MD PhD, Associate Professor of Radiology, at the David Geffen School of Medicine, UCLA, California
With advances in radiology over recent years medical imaging has become more precise, meanwhile even allowing for functional and metabolic analysis. It can be expected that imaging with genetic and molecular markers will play an important role in the foreseeable future. New developments in contrast media research, with the design of biologically specific contrast agents for a variety of imaging techniques, and for magnetic resonance imaging in particular, are expected to further contribute to the accuracy of medical imaging.
Molecular imaging may be characterised as the in vivo visualisation of specific biological processes at the cellular or even molecular level. It aims at the detection of early underlying biochemical and genetic alterations responsible for various disease entities rather than late changes as demonstrated by most current diagnostic imaging tools.
Excellent soft tissue contrast, high anatomical resolution and multiplanar imaging capabilities qualify MRI for molecular imaging. Compared with positron emission tomography (PET), with or without computed tomographic imaging (PET/CT), MRI does not require coregistration of molecular activity with anatomical structures. In addition, the lack of radiation exposure further favours the use of MRI. However, relatively large and potentially toxic concentrations of imaging markers are commonly necessary to visualise molecular events, representing some of the challenges faced by molecular MRI.
For a successful implementation of molecular MRI the following criteria should be fulfilled:
• sufficient MR contrast to depict changes on a cellular or molecular level
• favourable safety features of the contrast agent / molecular probe; and
• usefulness of the probe with proven validation for basic science or clinical application.
The current probes for molecular MRI combine either a paramagnetic or superparamagnetic contrast compound with a ligand. The ligand binds with high affinity to a molecular target or receptor, which usually serves as an imaging biomarker, allowing assessing the presence or severity of disease. Targets may range from copies of DNA to multiple intra- or extracellular proteins or metabolites. The capacity of a probe to detect the target molecule follows the rules of classic pharmacology. Therefore, the route of administration, distribution and delivery to the target, followed by elimination through metabolism or excretion, need to be considered. As the ligand-receptor interaction is dynamic in nature, the correct timing of the imaging data acquisition is crucial. Usually molecular contrast agents require a 24-hour period after administration until a significant amount of the probe has accumulated at the target to provide sufficient signal for MR imaging. Therefore, registration of pre- and postcontrast data sets is usually required. Image resolution and speed of data acquisition are further important determinants for the depiction of signal changes.
A substantially different means of imaging molecular events is MR spectroscopy. This relies on the detection of a spectroscopic peak at a certain location, generated by a metabolite that is produced by, or heralds, alterations on a molecular level.
Clinical Applications of molecular MRI include oncologic imaging, detection of thrombosis as well as imaging of inflammatory and/or rheumatoid disease. In addition, genetic and cell-based therapies are likely to benefit from molecular imaging.
Molecular MRI holds promise to enhance tumor detection, to provide accurate staging, to monitor therapeutic response and to survey for recurrent disease. The primary goal in oncologic imaging is to improve the detection of malignant cells, both at the primary site of origin and at locations of metastasis. As a common characteristic tumor growth is paralleled by the de novo formation of blood vessels. MRI probes aimed to specifically detect molecules mediating the process of angiogenesis have been employed to assess tumor growth and malignant potential.
Similar to FDG-PET, an imaging modality which measures increased tumor metabolism to highlight areas of tumor, a contrast agent based on (Gd)-encapsulated liposomes has been developed with ligands bearing glucose conjugates at the liposome surface. Targeting of tumor cells with liposomes is attractive for two reasons. Firstly, high concentrations of the MRI contrast material can be delivered in the liposomes, secondly, liposomes can be used to deliver drugs. Thus, the simultaneous detection of malignancy and delivery of chemotherapeutic agents appears feasible. Possible drawbacks, however, include the relatively large size of the liposome challenging the access into the extracellular compartment, and the immunogenic potential of the liposomes.
An approach to monitor tumor progression and response to treatment has been developed based on a superparamagnetic probe specific to cells expressing a molecule (Synaptotagmin I) that binds to apoptotic cells. Since the degree of programmed cell death after radio or chemotherapy has been shown to correlate with impediment of tumor growth and cure, the superparamagnetic compound conjugated to synaptotagmin showed good correlation with apoptosis both in vitro and in vivo.
Potential benefits of lymphotropic superparamagnetic nanoparticles have been extensively discussed in the radiological and oncologic literature. They show the potential for widespread clinical application in order to stage nodal malignant disease, particularly in the presence of prostate cancer. Clinical investigations have demonstrated that MRI with superparamagnetic nanoparticle contrast agents may improve the diagnostic accuracy. The application of these iron-based contrast agents allow the detection of malignant lymph nodes smaller than the threshold size of 10 mm that is commonly used to identify nodal disease on conventional cross-sectional imaging.
In addition to oncologic applications molecular MRI based on the use of superparamagnetic iron particles has been investigated for the diagnosis of inflammatory disease such as in antigen-induced arthritis or for the early detection of atherosclerotic disease. It has been shown that activated macrophages, which are part of the disease process, can be labeled with iron particles, presumably by macrophage phagocytosis. These initial results suggest that molecular MRI might play a role for the advanced detection of inflammatory disease, the characterisation of the degree of macrophage infiltration, and to monitor treatment response.
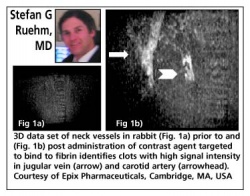
Current MR techniques aimed at the detection of arterial or venous thrombosis would benefit from a more specific approach to depict clot. As many clinically significant thrombotic events occur in small arteries that are below the resolution of current fast MR sequences, for example distal coronary vessels or peripheral pulmonary arterial branches, a targeted probe to provide a specific marker to improve the detection of small thrombi would be desirable.
With the development of clinical applications for gene therapy molecular MRI may play an important role for monitoring and quantifying the amount of gene delivery to an area of interest and to survey the efficiency and duration of gene expression. For that purpose ‘intelligent’ molecular imaging probes have been developed. In the presence of a common reporter gene product such as _-galactosidase (_-gal), these probes undergo an irreversible transition from a weak to a strong relaxivity state. Cells that express the therapeutic gene generally also express the reporter gene _-galactosidase. Thus a high signal detectable by MRI is generated.
Molecular MRI may also permit quantification and localisation of gene expression. For this purpose the transgene for tyrosinase has been incorporated into cells. The activity of the transgene can then be measured by its production of melanin. Melanin reveals a high binding capacity for metal. It has been shown that as a result of the tyrosinase expression bright signal of iron containing melanin can be detected on T1-weighted MR images.
Comparable to gene-therapeutic approaches, cell-based therapies are used with increasing frequency. Molecular MRI might provide substantial support to establish these recent treatment options by offering strategies to monitor the success of treatment. Bone marrow transplantation is widely employed and might benefit from in vivo tracking of transplanted haematopoietic cells, particularly when modified treatment regimens are evaluated. Transfection techniques allow increasing the amount of contrast agent delivered to cells. This enables even the visualisation of single cells. With the further advancements in the development of cell-based therapies, e.g. the transplantation of cardiac myocytes to restore myocardial function, the demand for specific and non-invasive imaging strategies is likely to increase.
The enormous potential for molecular MRI is based on evidence that most diseases are caused by alterations on a molecular level that may be detected by sophisticated imaging modalities. In contrast to current diagnostic imaging algorithms molecular MRI moves far beyond the structural foundation of traditional imaging techniques. Molecular MRI aims to reveal the biochemical and genetic sources of the disease in addition to the mere display of anatomy and physiology. The synergy of refined MRI techniques and new, targeted contrast agents will determine the success of molecular MRI in the future. It is likely that molecular MRI will open a wide range of novel applications, not only for the assessment of disease, but in screening for pre-disease states, which might be commonplace in the upcoming era of genetic medicine. In the consumer-driven health care environment imaging faces a bright future. However, more research is necessary to prove the value of these high tech imaging techniques with regard to overall patient outcome and cost.
Links: sruehm@mednet.ucla.edu
07.08.2006