Digital pathology
The challenges of digital pathology
In order to be able to properly locate digital pathology in the current discourse on the digitalisation of healthcare, pathology has to be understood as a fundamental diagnostic discipline, above all in oncology.
Report: Stefan Kropf


Any tissue sample taken in a surgical intervention is sent to the pathology lab: The tissue, placed on a slide and processed depending on the question at hand, e.g. stained, is examined under a microscope in order to detect pathological structures and changes which then can be treated in a targeted fashion along the clinical pathway.
In digital pathology the microscope is replaced by a slide scanner linked to a reading station where the image data are assessed. Thus the pathologist no longer "reads" the physical slide under a microscope but the digital images on a monitor.
There is no doubt that over the next few years digital pathology will move from niche to mainstream - too convincing are its benefits. Nevertheless, its drawbacks need to be addressed.
The most obvious advantage of digital pathology is the fact that the location of the reader - the pathologist - is no longer linked to the location of the physical tissue sample. Without the need to mail slide and sample, second opinions can already be obtained during initial assessment of the specimen. Furthermore, routine reading can be done from the home office so to speak.
A further advantage is the possibility to view the entire slide which enhances orientation within the specimen. Images with different stains can be superimposed to create better contrast of interesting structures. Image analysis software allows precise selection of the structures to be evaluated and notes can be added right on the image and saved.Beyond these advantages research and teaching provide ample opportunity to use digital images.
The disadvantages of digital pathology are expensive equipment, instable technology, lack of standards and lab information systems that are not yet ready to support digitalization.
With a view towards digital pathology current lab information systems need to address certain issues - issues that go beyond digital pathology but are relevant for the digitalisation of healthcare as such:
- internal and external order entry
- secure platforms for the exchange of image and document
- unified formats, such as DICOM, and open hardware interfaces to integrate different devices
- integration and processing of legacy data (reports)
- labelling and assignment of procedures and processes
- integration of different devices (tablet PCs, smartphones, VR headsets) to facilitate reading/reporting and communication with clinicians (e.g. tumour board)
- creation of communication pathways between pathologists and clinicians
- automatization of data input and transfer, e.g. ADT/GEKID
- molecular pathology and its information and diagnostic potential
When the future requirements of an LIS are defined from the vantage point of the core task of digital pathology - to make histological specimens available in a digital format - the issues industry has to tackle become clear. And indeed industry has its work cut out to achieve stable and mature systems.
In order to be able to digitalize this core task the process of pathological assessment first has to be described analogically.
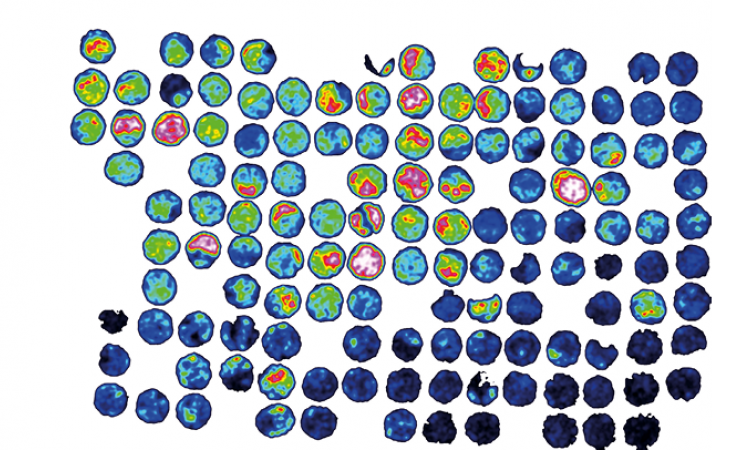
In conventional diagnostics the pathologist uses a microscope - as well as his or her experience - to assess tissue morphology, cell size, cell distribution and colour. The patterns the pathologist detects allow a diagnosis - or at least point in a diagnostic direction. These parameters can be mapped in digital pathology. Case in point: staining. The different colours illustrate the potential of assessing digitalized slides. Calibrated systems can perform these assessments and provide probability values as percentages, thus paving the way towards diagnosis. Task lists based on "regions of interest" can be created, prioritized and distributed to several pathologists. Moreover, the probability values can trigger further examinations, for example in molecular pathology, that lead to personalized healthcare.
The major challenge with regard to digital mapping of an analogue process is the correct handling and categorization / allocation of data. On the one hand there are ancillary tasks which nevertheless require a high degree of "tweaking" into digitalization, such as data capture, creation of cases, data transfer and report shipping. On the other hand there are the data to be managed in the LIS - and it is exactly these data that pose the major challenge since the LIS lacks the crucial feature of the human pathologist: diagnostic experience. Any digital system will have to close this knowledge gap. Thus, every kind of information needs to be validated by a specialist prior to integration in the system. Moreover it is not only the quantity but also the quality of information that determines the level of detail of subsequent diagnostic procedures. These developments are comparable to the digitalisation of radiology departments some years ago.
Conclusion
Pathology, indeed healthcare in general, is facing the enormous task of mastering the challenges digitalisation will pose in the coming years. Personalized medicine and "big data" will remain nothing but buzz words if the information systems don't manage to meet the requirements of digitalisation. At the same time we should not forget that the new possibilities will also create new dependencies. They need to be addressed in order to avoid a complete breakdown of the diagnostic tasks that are performed every single day. Thus, any new pathology LIS solution needs to offer a reliable disaster recovery strategy, including the post-failure input of analogue data. Even in the digital future the pathologist has to be able to dust off and use the microscope as analogue assessment tool. In short: digitalisation supports the pathologist's assessment - it does not replace it!
02.08.2016