Image source: Liu et al., Nature Communications 2021 (CC BY 4.0)
News • Hydrogel framework
Synthetic tissue with growing blood vessels developed
Using lab-created tissue to heal or replace damaged organs is one of the great visions for the future of medicine. Synthetic materials could be suitable as scaffolding for tissue because, unlike natural tissues, they remain stable in the organism long enough for the body to form new natural structures.
A fundamental requirement for functional tissue is that blood vessels must be able to grow in them and connect to the organism’s vascular system, so that the tissue is properly supplied with oxygen and nutrients. However, until now, almost nothing has been known about which material properties promote the growth of blood vessels. A team headed by biomedical engineer Dr Britta Trappmann from the Max Planck Institute for Molecular Biomedicine in Münster, Germany, has developed a cell culture system in which, for the first time, a functional blood vessel system is able to grow within a framework made of synthetic materials. The study was now published in the journal „Nature Communications“.
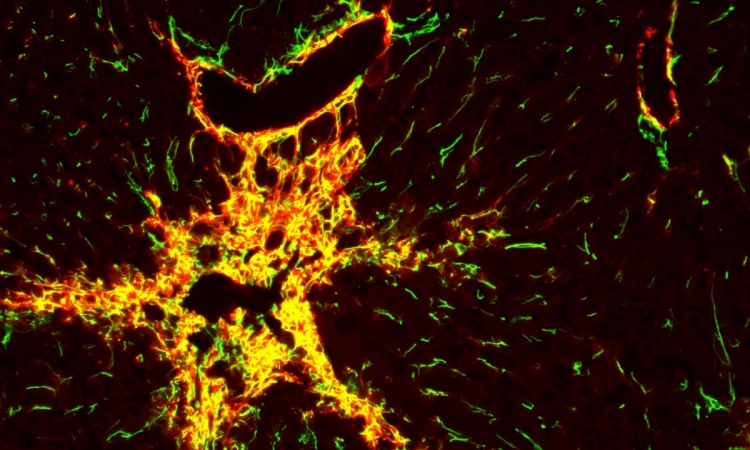
The endothelial cells form contacts with each other and attach to their synthetic tissue environment in the channel, thus forming a parent blood vessel after about a day
Britta Trappmann
The scientists, working in a special hydrogel with properties they can change in a controlled manner, first grew a parent blood vessel from human blood vessel lining cells. They then investigated how the material properties of the artificial cell environment influenced the formation of additional blood vessels and fine-tuned them. Summarizing the key findings, Britta Trappmann highlights that “The synthetic tissue material must activate certain adhesion molecules in the membrane of blood vessel cells so that the cells migrate in groups from the parent vessel and form tubular structures. At the same time, the material must be sufficiently degradable for the cells to form blood vessels of adequate size”. In order to mimic the natural environment of cells, many additional biomolecules and cells would have to be integrated into the model system in later steps – these may be signaling proteins, immune cells or cells to stabilize the blood vessels. “Moreover, the effect of all these factors is linked in natural tissues and varies from organ to organ,” Britta Trappmann explains. Understanding all of this, she says, is a long-term goal but, ultimately, the knowledge might then be used to grow implantable tissues. In their investigations, Britta Trappmann and her team worked together with colleagues at the University of Münster as well as working groups from Munich and North Carolina.
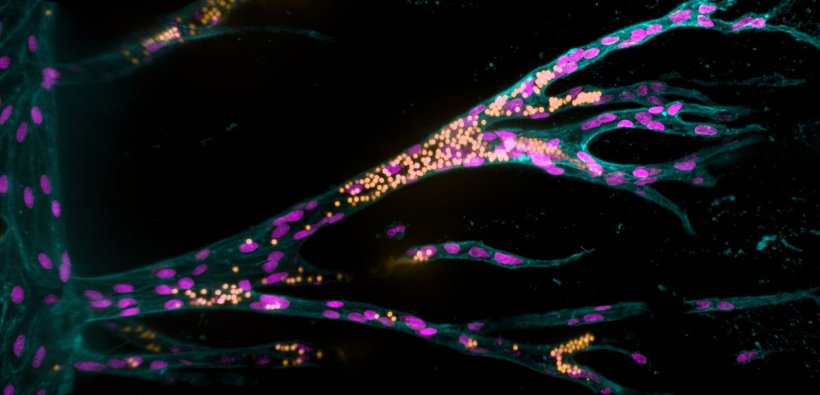
In this study, researchers refined a model system that Britta Trappmann developed with colleagues during her time as a postdoc in the USA at Boston and Harvard Universities. It consists of a three-dimensional sugar-based hydrogel into which the scientists make two channels using an acupuncture needle. Each channel has a diameter of 400 micrometres and they run parallel to each other at a distance of approximately one millimetre. In one channel, the scientists seed endothelial cells, which line blood vessels in natural tissues. “The endothelial cells form contacts with each other and attach to their synthetic tissue environment in the channel, thus forming a parent blood vessel after about a day,” explains Britta Trappmann. When this has happened, the scientists deliver a growth factor cocktail of molecules that drive blood vessel growth in natural tissues through the second channel, whereupon the endothelial cells migrate into the hydrogel.
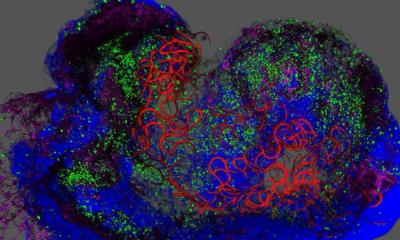
The scientists then wanted to find out which properties of the hydrogel determine whether the migrating endothelial cells actually form new blood vessels. They investigated the role played by the activation of so-called adhesion molecules in the cell membrane through which cells adhere to their surrounding environment. The researchers first enriched the hydrogel tissue framework with varying amounts of peptides that activate a certain type of adhesion molecule found in the membrane of endothelial cells called integrins. The higher the concentration of peptides, the more the endothelial cells migrated together through the hydrogel. In contrast, when the scientists blocked integrin function they observed that the cells only migrated individually. In a further step, the team investigated this process looking at two specific integrin subtypes. “We found that integrin αvβ3 is the crucial adhesion molecule that must be activated for endothelial cells to migrate in groups,” Britta Trappmann says. The scientists also showed that collective cell migration is, in turn, a prerequisite for the endothelial cells to form cavities connected to the parent vessel in the next step.
Although the blood vessel cells then formed tubular structures, these were smaller than those in natural tissues. The scientists hypothesized that this could be because the synthetic hydrogel is less degradable than natural tissue and has smaller pores through which the cells can slip. As the hydrogel consists of sugar molecule chains that are crosslinked by certain molecules, the scientists’ solution was to exchange these crosslinker molecules so that the cells could cleave the hydrogel more quickly using the enzymes they release. This allowed the cells to migrate faster and form larger vascular structures.
Source: Max Planck Institute for Molecular Biomedicine
28.07.2021