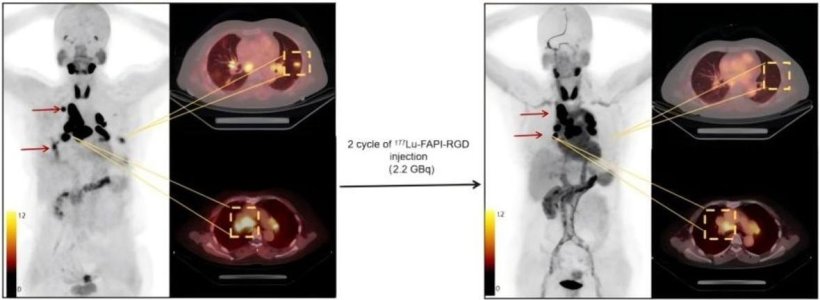
One representative patient with a favorable therapeutic response after 2 cycles of 177Lu-FAPI-RGD. A 56-year-old woman with disease progression after surgery received 177Lu-FAPI-RGD RLT at 2.2 GBq/cycle. Baseline 68Ga-FAPI-RGD PET/CT revealed intense 177Lu-FAPI-RGD uptake in most metastatic lesions including lymph node and lung modules (left, arrows and square box). After 2 treatment cycles, restaging 68Ga-FAPI-RGD PET/CT revealed a significant reduction in tumor size in most of the metastatic lesions. Furthermore, most of the lesions in the lungs have disappeared (right, arrows and square box).
Image source: SNMMI; from: Xiang J, Wang R, Wang J et al., Journal of Nuclear Medicine 2025
News • Dual targeting radioligand
“Smart missile” radiopharmaceutical shows promise against multiple cancers
A new cancer treatment that delivers radiation directly to tumors by targeting two key markers simultaneously has been shown to be safe and effective in human trials.
Acting like a “smart missile,” the dual-targeting radiopharmaceutical is designed to attach to two vulnerable sites on cancer cells, enabling more precise and potent therapy. Early results show that nearly 90% of patients experienced either tumor shrinkage or disease stabilization. These findings were presented at the Society of Nuclear Medicine and Molecular Imaging 2025 Annual Meeting.
“Radiopharmaceutical therapy is a promising new tool for anti-tumor treatment, and is already used clinically, most notably for prostate cancer and neuroendocrine tumors,” said Jialing Xiang, PhD student at Peking Union Medical College Hospital in Beijing, China. “While most existing therapies target a single molecular biomarker, my colleagues and I explored a dual-targeting strategy to simultaneously engage two tumor-associated markers, with the aim of enhancing tumor uptake and therapeutic efficacy across multiple types of cancer.”
The beauty of this drug is that it stays in tumors for days, allowing prolonged damage to cancer cells
Jialing Xiang
This first-in-human study assessed preliminary safety, biodistribution, and dosimetry of 177Lu-labeled FAPI-RGD, the combined radiopharmaceutical therapy drug. Nine patients with advanced adenocarcinomas—including pancreatic, pulmonary, renal, and ovarian cancers—received a single treatment cycle of the investigational agent. Baseline uptake was confirmed using 68Ga-FAPI-RGD PET/CT imaging, which also assessed dynamic distribution throughout the whole body. Safety evaluations were conducted and a repeat 68Ga-FAPI-RGD PET/CT scan was performed after each treatment cycle to assess early response.
Treatment with 177Lu-DOTA-FAPI-RGD was well tolerated by all patients, with no noticeable side effects reported. The therapy showed strong and lasting uptake in tumors, allowing for a high dose of targeted radiation to be delivered directly to cancer cells. In total, 88.9% of patients responded positively, with their cancer either shrinking or stopping its growth. Many also experienced relief from physical symptoms—reporting less pain, better appetite, and an overall improvement in quality of life compared to previous treatments.
“The beauty of this drug is that it stays in tumors for days, allowing prolonged damage to cancer cells,” noted Xiang. “It offers real hope for patients with advanced cancers who have exhausted other treatment options. Future prospective trials are planned to optimize dosage for maximum patient benefit, and we look forward to promising developments ahead.”
Source: Society of Nuclear Medicine and Molecular Imaging
25.06.2025