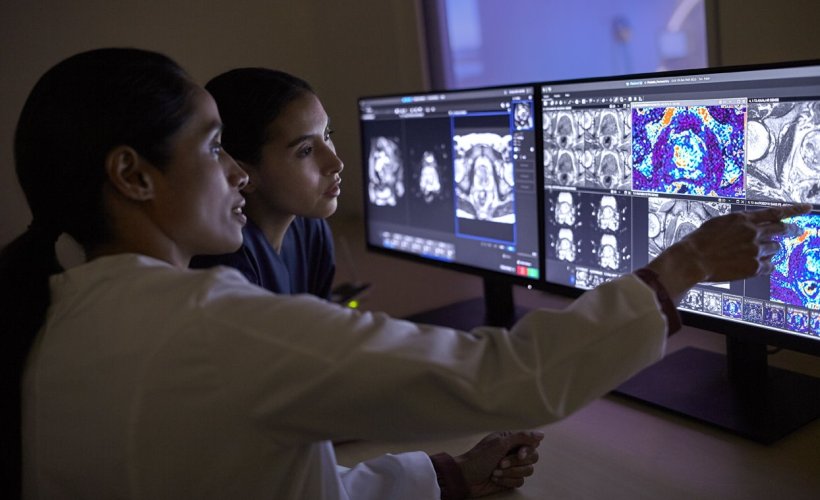
Image source: Philips
News • AI-based imaging and reporting solutions for MRI
Philips partners with imaging biomarker specialist Quibim
Combination of Philips’ leading AI-enabled MR imaging and Quibim’s AI-enabled image analysis software enables clinicians to deliver faster, easier prostate cancer care, the company reports.
Prostate cancer has a significant impact globally. According to the American Cancer Society, approximately one in eight men in the US will be diagnosed with prostate cancer at some point in their lifetime.1 Early detection currently relies largely on a prostate-specific antigen (PSA) screening blood test that may help detect prostate cancer early, especially for slow-growing cancers that never spread beyond the prostate gland. However, PSA screening tests also have poor specificity to clinically significant cancer, which can lead to many false positives and overdiagnoses. As a result, patients undergo painful biopsies that turn out to be negative, resulting in significant anxiety as they wait for their biopsy results complicated by an avoidable pathology workload.
While MR exams are generally more expensive and time-consuming than PSA tests, recent research indicates MR may provide value as a triaging tool to guide biopsy decisions, as well as a valuable diagnostic tool to help determine treatment planning and personalized therapy independent of PSA testing.2 By reducing the number of unnecessary biopsies performed and enabling better-targeted therapy for prostate cancer that does need treatment, MR exams can potentially result in overall cost savings and faster, more accurate diagnoses for patients.
Recommended article
Article • Research, diagnostics, therapy
Focus on prostate cancer
Prostate cancer (PCa) is not only one of the most common, but also one of the deadliest types of cancer in men. Diagnostics are correspondingly sophisticated, from imaging via ultrasound or MRI to various biopsy techniques – often even in combination. Keep reading for current developments in early detection, staging, therapy and research.
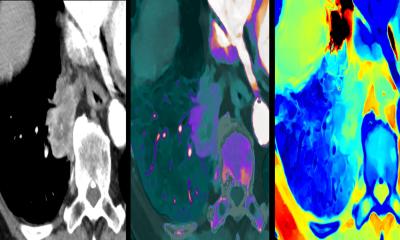
Philips is already leveraging AI-powered technology like MR SmartSpeed image reconstruction software, which helps build clinical confidence with up to 65% higher resolution and outstanding image quality.3 Philips and Quibim will now work on an integrated solution including Quibim’s artificial intelligence (AI) based QP-Prostate software,4 to automate real-time prostate gland segmentation in MR images5, generating meaningful quantitative insights, and standardizing MR prostate exam reporting. Combined with Philips’ AI-based MR imaging techniques, the solution aims to provide clinicians with the speed and precision needed to deal with the growing number of patients in need of MR exams to help enhance diagnostic confidence, personalized treatment and better outcomes.
This integrated approach, combined with workflow enhancing features, will help mitigate staff shortages, high burn-out rates, and cost constraints currently being experienced by many radiology and oncology departments
Angel Alberich-Bayarri
“This collaboration with Quibim is the latest example of our commitment to build an AI ecosystem into our Diagnostic Imaging portfolio to help detect conditions like cancer earlier, improve the rate of accurate first-time-right diagnosis, and streamline hospital operations to provide better care at lower costs,” said Ruud Zwerink, General Manager of MR at Philips. “The collaboration with Quibim will ultimately be extended to address other forms of cancer beyond prostate, where there is a need to improve efficiency and mitigate staff shortages while delivering high quality oncology care to an increasing number of patients.”
“The collaboration including Philips’ high-speed MR imaging and Quibim’s QP-Prostate software aims to provide the speed and diagnostic confidence to support all the different steps in an integrated diagnosis, treatment, and therapy assessment workflow. The upcoming version of our lesion detection algorithm will further expand the possibilities of MRI as a gamechanger in prostate cancer screening,6” said Angel Alberich-Bayarri, CEO of Quibim. “This integrated approach, combined with workflow enhancing features, is aimed at mitigating staff shortages, high burn-out rates, and cost constraints currently being experienced by many radiology and oncology departments. Patients will also greatly benefit from far less complex and painful biopsy procedures7 and more personalized treatment.”
Recommended article

Article • Image analysis in radiology and pathology
"The time has come" for AI
AI has made an extraordinary qualitative jump, particularly in machine learning. This can help quantify imaging data to tremendously advance both pathology and radiology. At a recent meeting in Valencia, delegates glimpsed what quantitative tools can bring to medical imaging, as leading Spanish researcher Ángel Alberich-Bayarri from imaging biomarker company Quibim unveiled part of his work.
“Because of its strong sensitivity to aggressive tumors, MRI has become the cornerstone of the prostate cancer diagnostic pathway. We now need to improve its specificity to avoid unnecessary biopsies, and its inter-reader reproducibility to allow reliable diagnoses outside of expert centers,” said Prof. Oliver Rouvière, Head of the Department of Radiology, Hôpital Edouard Herriot, Lyon. “Two of the biggest challenges facing any AI-software dedicated to prostate MRI include the ability to demonstrate good specificity, while maintaining a high level of sensitivity. Above all, we need to deliver robust diagnostic outcomes across different imaging protocols, magnetic field strengths and vendors."
Philips MR solutions and Quibim’s QP-Prostate software will be demonstrated in the Philips booth (#6730) and the Quibim Booth (#6336) at the Radiological Society of North America Annual Meeting (RSNA 2023, November 26-29, Chicago, USA).
References:
- American Cancer Society, Key Statistics for Prostate Cancer
- Moore CM et al.: Prevalence of MRI lesions in men responding to a GP-led invitation for a prostate health check: a prospective cohort study; BMJ Oncology 2023
- Compared to Philips SENSE
- QP-Prostate is an FDA 510k cleared solution and a UKCA and CE mark (Class IIb) cleared solution under MDR.
- Jimenez-Pastor A et al.: Automated prostate multi-regional segmentation in magnetic resonance using fully convolutional neural networks. European Radiology 2023
- Automated lesion detection is pending regulatory clearance and is currently only available as a research-only solution.
- Elwenspoek MMC et al.: Comparison of Multiparametric Magnetic Resonance Imaging and Targeted Biopsy With Systematic Biopsy Alone for the Diagnosis of Prostate Cancer: A Systematic Review and Meta-analysis. JAMA Network Open 2019
Source: Philips
27.10.2023
- AI (822)
- biomarkers (177)
- cooperation (357)
- imaging (1638)
- MRI (832)
- prostate (30)
- prostate cancer (219)