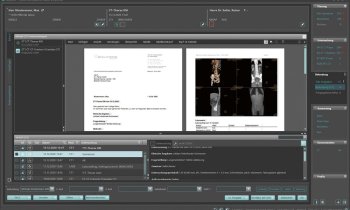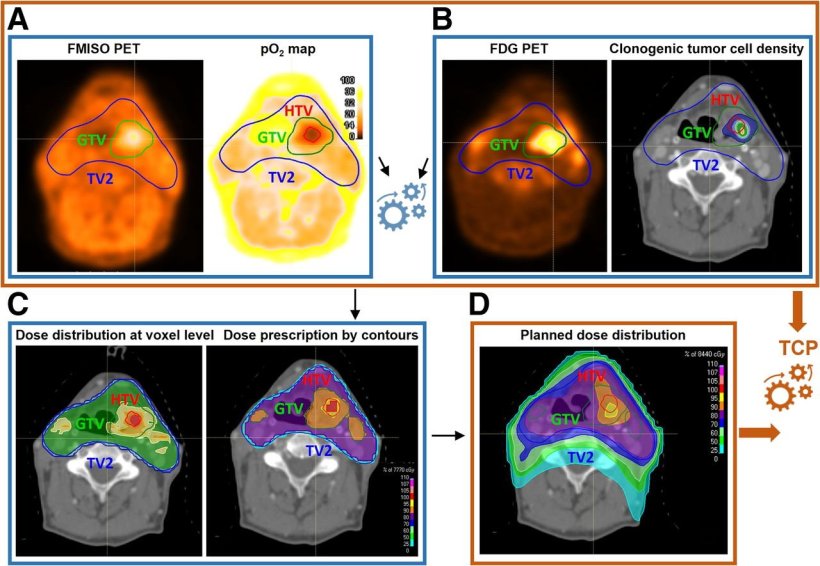
Study workflow. (A) [18F]FMISO PET was used to derive voxel-level pO2 maps. (B) [18F]FDG PET provided information on clonogenic tumor cell distribution. These datasets informed voxel-level dose prescription to counteract radioresistance, determining required dose escalation for hypoxic volume (C). Resulting planned dose distribution (D), together with radiosensitivity and clonogenic cell density maps, was used to predict TCP. Color scale in pO2 map in panel A shows oxygen distribution (range, 0 100 mm Hg), whereas color scales in panels C and D show percentage of maximum dose in treatment plans.
Image source: Lazzeroni M, Ureba A, Schäfer H et al., Journal of Nuclear Medicine 2025 (CC BY 4.0)
News • Two tracers, one target
Dual PET approach for personalized head and neck cancer treatment
Novel imaging strategy could push tumor control rates from 60% to over 90%
A new strategy that combines two types of PET scans can guide personalized radiotherapy for head and neck cancers, according to new research published in The Journal of Nuclear Medicine. Moving beyond the "one-size-fits-all approach," this research shows that treatment can be biologically tailored in a clinically feasible way with the potential to improve patient outcomes.
Conventional radiotherapy prescribes the same dose for nearly all patients based on the type of their tumor and standard tissue anatomy. Advances in PET imaging, however, allow physicians to create biologic maps that show the unique characteristics of a tumor and can be utilized to develop a personalized treatment plan.
The results from this proof-of-concept study highlight how molecular imaging can play an active role in guiding treatment decisions, not just diagnosing disease
Marta Lazzeroni
"This study shows a new way to personalize radiotherapy using two different PET tracers in the same patient. In a novel approach, we combined PET imaging of tumor oxygen levels, which influence radiation resistance, with PET imaging of tumor cell density, and used this information to calculate how much radiation dose is needed in different tumor regions," said Marta Lazzeroni, PhD, associate professor of Medical Radiation Physics at Stockholm University in Sweden.
Researchers explored a biologically guided treatment strategy for head and neck squamous cell carcinoma (HNSCC), using 18F-FDG PET to estimate relative cell density and 18F-FMISO PET to quantify hypoxia-related radioresistance. Twenty-eight patients received both scans, and cellular information was analyzed to create individual tumor profiles. These biologic maps informed personalized treatment plans, with escalated doses designed for regions of the tumors with unfavorable characteristics.
Planned dose distributions achieved greater than 90% predicted tumor control probability based on radiobiological modeling in all cases, a significant increase from the approximately 60% tumor control probability reported in previous literature. All treatment plans met standard clinical feasibility criteria to protect healthy organs and tissues, demonstrating the overall feasibility of the personalized strategy.
"The results from this proof-of-concept study highlight how molecular imaging can play an active role in guiding treatment decisions, not just diagnosing disease," stated Lazzeroni. "In the future, PET imaging could become a key tool for designing truly personalized radiotherapy treatments and for adapting therapy as tumors change over time."
Source: Society of Nuclear Medicine and Molecular Imaging
01.02.2026