© Choi_ Nikolai – stock.adobe.com
News • Weight loss and diabetes medication
GLP-1 agonists may interfere with cancer imaging, new study finds
The growing use of GLP-1 receptor agonists may affect the interpretation of oncological FDG PET/CT scans, new research presented at the 38th Annual Congress of the European Association of Nuclear Medicine (EANM'25) has revealed.¹
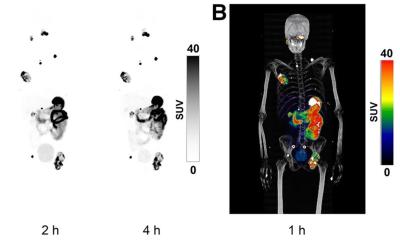
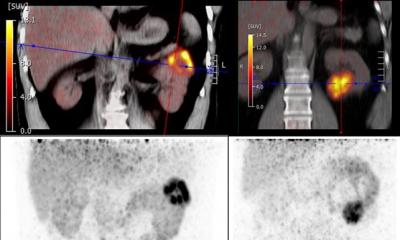
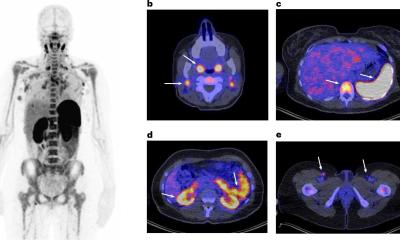
GLP-1 receptor agonists are now widely prescribed for individuals with type 2 diabetes and weight loss, with a 700% increase in usage reported in the United States between 2019 and 2023.2 These medications alter glucose metabolism, gastric motility and sympathetic tone, which may lead to unique uptake patterns on PET/CT. Previous case reports have shown increased FDG uptake in skeletal muscle, myocardium and brown adipose tissue, findings that may be mistaken for malignancy or inflammatory disease.3,4
Researchers from Alliance Medical Ltd. performed a retrospective case series review of oncologic FDG PET/CT scans in patients taking GLP-1 agonists. They observed several atypical patterns of tracer uptake that could be misinterpreted as pathology if a patient’s medication history is not considered. “We noticed unusual uptake in one of our patients on a GLP-1 agonist, which prompted a wider review across our network,” explained lead author Dr Peter Strouhal, Medical Director at Alliance Medical Ltd. “We found that these altered patterns are increasingly common, yet there is currently no national or international guidance in the UK addressing this emerging issue.”
Recognising the characteristic uptake associated with GLP-1 agonists helps avoid unnecessary anxiety and interventions, ensuring patients receive the right care, at the right time, without detours or doubt
Peter Strouhal
Misinterpreting these uptake patterns can lead to unnecessary investigations, inappropriate cancer staging and delays in treatment, which may cause stress and uncertainty for patients. “Recognising the characteristic uptake associated with GLP-1 agonists helps avoid unnecessary anxiety and interventions, ensuring patients receive the right care, at the right time, without detours or doubt,” Dr Strouhal added.
At present, the researchers do not recommend altering patient preparation or stopping GLP-1 agonists prior to FDG PET/CT scans. Instead, they advise that imaging teams carefully document patients’ medication histories to inform interpretation while formal guidance is developed. Current UK guidelines do not address this issue, although Australian guidance suggests continuing treatment, fasting from midnight, scheduling morning scans and ensuring good glucose control.5
The research group intends to extend their data collection across additional imaging centres to provide a stronger evidence base for future national guidance. They also aim to establish international collaborations so that patients everywhere benefit from consistent and reliable PET/CT interpretation.
References:
- Strouhal P, Meadows A, McGovern A. A Weighty Problem: GLP-1 Agonists and the Altered Images of FDG PET-CT. Presented at EANM'25 on Wednesday 8 October 2025.
- Mahase E. GLP-1 agonists: US sees 700% increase over four years in number of patients without diabetes starting treatment. (2024.) BMJ.
- Oldan JD, Landman PG, Schroeder JA et al.(2024). FDG PET in a Patient on a GLP-1 Agonist/Insulin Secretagogue. Clinical Nuclear Medicine.
- Harrison DB, Phillips AL, Tansey JB et al. (2025). Brown Adipose Tissue Mimicking Head and Neck Cancer on PET Scan in a Patient on GLP-1 Drug. Laryngoscope. PubMed. National Library of Medicine.
- Greenfield J, Mikaheal Y and Ludington J. (2024). Joint ADS/ANZSNM guideline for FDG PET/CT imaging in patients with type 1 and type 2 diabetes. Australian Diabetes Society & ANZSNM.
Source: European Association of Nuclear Medicine
08.10.2025