Interview • International research collaboration
The Pan-Cancer project: Cancer development begins within the genes
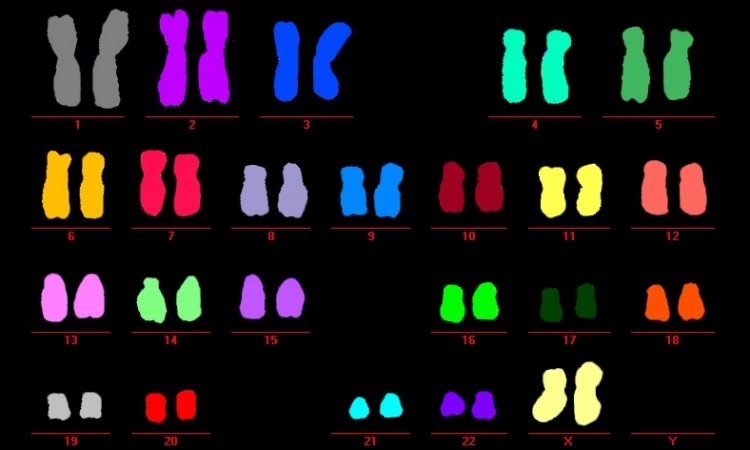
Launched in 2011, the Pan-Cancer Project, involved more than 1,300 scientists and clinicians in 37 countries, and analysed more than 2,600 genomes of 38 tumour types. Discovery: The first indications of cancer development can be found in genes at a very early stage, which triggers new opportunities for treatment.
Interview: Katrin Schreiter
Within the project, Leipzig-based scientists specifically analysed the DNA material. Initially in our interview, Dr Hans Binder, Managing Director and research leader at the Interdisciplinary Centre for Bioinformatics (IZBI) at Leipzig University explained how the cancer cells examined.

‘Doctors took tissue samples from patients and diagnosed the cancer. Pathologists examined the tissue to make further, detailed diagnoses and laboratory medics and molecular biologists extracted the DNA and sequenced it. The sequence data provided information about the mutations. We have developed bioinformatical methods and used them to derive mutations and further information such as activity of the genes and possible problems with biological functions from the sequence data. This makes it possible to differentiate between types of cancer through a comparison of the nucleotide sequences along the DNA, or respectively the frequency of RNA copies read out in samples from the cancer patients.’
The findings: ‘With our partners across Germany we contributed towards a better understanding of lymphoma, which mechanisms lead to its origin and development; why the affected cells degenerate… yes, this was a vast amount of data involving millions of sequence positions in around 20,000 genes, from hundreds to a few thousand patients respectively, which the bioinformaticians prepared in such a way that conclusions can be drawn.’
Can those conclusions be generalised?
‘Cancer is a complicated disease which develops because of defects in the genome. Strictly speaking, with each patient it is not a disease but actually a multitude of defects which can have different effects. For the first time, the project gave an overview of the possible defects.
‘Unfortunately, a genetic defect cannot simply be repaired, as side effects of treatment are not predictable and controllable. This makes the disease so difficult. But research is progressing. It is important to learn how cancer works. We have already developed improved diagnosis- and treatment options, such as immunotherapy. Apart from fighting cancer we also need to adjust treatment in such a way that patients can live with the disease as best and for as long as possible.’
Have you gained any insights on how cancer presents in the initial stage?
‘On a DNA level you mostly find so-called driver mutations. This often affects genes with key functions and leads, for instance, to increased cell division, i.e. the cells infiltrating healthy tissue. Some of these changes can be detected in the genes, decades before the disease manifests. But simple and standardised treatment is not possible: Mutations make the cancer very variable; it adapts and becomes hard to fight.’
This sounds like a multitude of cell changes.
Each person’s genome is unique – which is why every cancer, as a disease of the genome, is also unique
Hans Binder
‘Yes, the variety is enormous. The important thing is knowing that each person’s genome is unique – which is why every cancer, as a disease of the genome, is also unique. However, the variants can often be grouped to devise specific treatment rules.’
Are these insights a further step towards personalised medicine?
‘Most definitely. It will be the responsibility of Cancer Research Centres (and already is in parts) to analyse the DNA of each patient to understand the specific characteristics of their disease and then tailor their treatment accordingly.’
Will the CRISPR method become more important in this context?
‘CRISPR is a procedure used to precisely modify DNA building blocks in the genome. It works like genetic scissors. CRISPR is now of great importance for basic research, for instance to examine consequences of driver mutations in laboratory experiments. It’s too early for its use in therapy because collateral damage is not predictable. But there are other ways of fighting cancer.’
Recommended article

News • For the development of CRISPR/Cas9
Nobel Prize in Chemistry goes to Emmanuelle Charpentier and Jennifer A. Doudna
The Royal Swedish Academy of Sciences has decided to award the Nobel Prize in Chemistry 2020 to Emmanuelle Charpentier from the Max Planck Unit for the Science of Pathogens, Berlin, Germany, and Jennifer A. Doudna from the University of California, Berkeley, USA, “for the development of a method for genome editing”, more commonly known as the 'gene scissors' CRISPR/Cas9.
‘Cancer cells have the ability to adapt and hide. Therefore, the objective is to expose them to allow the body’s immune system to fight them, hence intensive research in immunotherapy – with the first success: One strategy is to remove the “invisibility cloaks” from the cancer cells, allowing the immune system to recognise and destroy them. Another strategy is the removal of patients’ immune cells to teach them to recognise cancer cells, then to inject these enhanced cells.’
The project has ended. What's next?
‘One of the objectives now is to implement the findings in clinical routine, to finetune diagnoses and circumvent resistance to treatment. A further project is aimed at understanding and avoiding the damaging side effects of immunotherapy. Molecular DNA analyses play a key role here.’
The Pan-Cancer projectt:
The international research team examined 2,700 cancer genomes from different types of cancer in this meta-analysis. The group of scientists also includes researchers from Leipzig University: Dr. Hans Binder, Managing Director and research group leader at the Interdisciplinary Centre for Bioinformatics (IZBI), Professor Markus Loeffler, Director of the Institute for Medical Informatics, Statistics and Epidemiology (IMISE), and Professor . Peter Stadler of the Institute for Informatics, Chair of Bioinformatics, and their teams.
They analysed sequence data of malignant lymphoma, i.e. cancer of the lymphatic system. This involved the detailed examination of DNA mutations, problems with DNA methylation and related changes in gene expression, i.e. gene activity. The Leipzig-based bioinformaticians contributed significantly to the understanding of the molecular causes of the disease and of its heterogeneity. They have been active in different areas of genome research of cancers for over a decade and are leaders in this field internationally. Work arising from the worldwide project will now be continued and centre around clinical implementations.
Profile:
Biophysicist Hans Binder has been Managing Director of the Interdisciplinary Centre for Bioinformatics at Leipzig University since its foundation in 2002. He leads the research group on ‘Omics, bioinformatics for health and systems biology’ at the IZBI. He focuses on transcriptome and genome data from sequence analyses to understand the molecular and systemic mechanisms of diseases, particularly cancer. His team develops bioinformatical procedures using machine learning, and is involved in several international and national research consortia.
02.11.2020