Overcoming multidrug-resistant cancer with smart nanoparticles
Multidrug resistance (MDR) is the mechanism by which many cancers develop resistance to chemotherapy drugs, resulting in minimal cell death and the expansion of drug-resistant tumors. To address the problem of resistance, researchers at the National Institute of Biomedical Imaging and Bioengineering (NIBIB) at the National Institutes of Health (NIH) have developed nanoparticles that simultaneously deliver chemotherapy drugs to tumors and inhibit the MDR proteins that pump the therapeutic drugs out of the cell. The process is known as chemosensitization, as blocking this resistance renders the tumor highly sensitive to the cancer-killing chemotherapy.
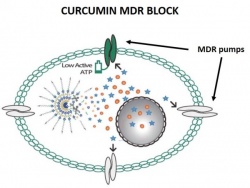
MDR is a major factor in the failure of many chemotherapy drugs. The problem affects the treatment of a wide range of blood cancers and solid tumors, including breast, ovarian, lung, and colon cancers. Researchers at NIBIB are engineering multi-component nanoparticles that significantly enhance the killing of cancer cells.
The work is led by senior author Xiaoyuan (Shawn) Chen, Ph.D., Senior Investigator, Laboratory of Molecular Imaging and Nanomedicine, NIBIB. His collaborators include scientists and engineers in China at Southeast University, Shenzhen University, Guangxi Medical University, and Shanghai Jiao Tong University, in addition to chemical engineers at the University of Leeds, United Kingdom.
Says Chen about his substantial network of collaborators, “success in this medically important endeavor has required a team with a wide range of expertise to engineer nanoparticles that survive the journey to the tumor site, enter the tumor, and successfully perform the multiple functions for chemosensitization.”
Targeting multidrug-resistant breast cancer
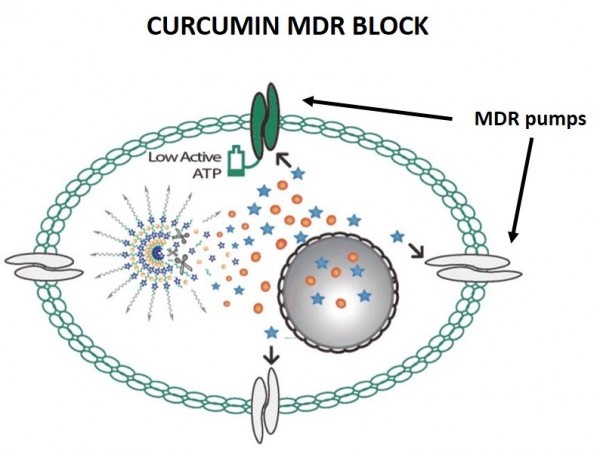
The two publications report on the engineering of two separate nanoparticles that test different strategies for achieving chemosensitization of cancer cells. The first targets MDR breast cancer. The engineered round nanoparticle is made of several layers. The center of the particle is loaded with the anti-cancer drug doxorubicin. The drug is surrounded by a water-repelling (hydrophobic) capsule to protect it from the watery environment when the particle is injected into the circulatory system of an experimental animal or individual with cancer.
The particle has several outer layers with different properties. One of the outermost components, a molecule called PEG, is hydrophilic (mixes with water) and helps the particle move through the bloodstream until it encounters the breast tumor cells. Another component on the surface of the particle, biotin, functions to bind specifically to the cancer cells and helps the drug-carrying nanoparticle to enter the cell.
Once inside the breast cancer cell, a fourth component called curcumin, which is intertwined with the doxorubicin center, is released along with the doxorubicin. The curcumin is the component that blocks the cell machinery that would pump the doxorubicin out of the cell. Without the ability to pump out the medicine, the cell is exposed to very high concentration of doxorubicin, which kills the breast cancer cells.
Experiments in mice demonstrated that the multi-component nanoparticles were effective at targeting breast tumor cells—accumulating at much higher concentrations in the cancer cells than in the other mouse tissues. Histology showed that the treated mice had a great reduction in cancer cell density in the tumor tissue compared with mice given saline or doxorubicin alone (not integrated into a nanoparticle). Complete analysis of the treated mice confirmed that the nanoparticle efficiently accumulated at the tumor site and achieved optimal tumor killing in the mouse breast cancer model.
Changing nanoparticle components tests alternate anti-cancer strategies
In the work published in Applied Materials & Interfaces, Chen and his colleagues describe the engineering of another nanoparticle that uses a different approach to the problem of MDR. This second nanoparticle is similar to the first in that it contains the centrally encapsulated doxorubicin surrounded by an outer hydrophilic surface layer that allows efficient transport through the bloodstream.
However, this particle uses the gas nitric oxide (NO), which is known to block the system that pumps doxorubicin out of the cell. In addition, the NO is released from a compound called BNN6, which is activated by ultraviolet (UV) light. Thus, this nanoparticle is designed to be administered in the bloodstream and then activated with UV light when it reaches its cancer target.
In experiments in cell culture, when hit with UV light the nanoparticles burst -- releasing the cell-killing doxorubicin and causing BNN6 to release the NO gas. The combination successfully inhibited the MDR machinery, resulting in chemosensitization and efficient cancer-cell killing. Based on the successful testing of this nanoparticle in cultured cells, the group expects it to perform well when tested in experiments in mice.
Smart nanomedicines vs multidrug resistance
Chemotherapy is the most common treatment for cancer. Unfortunately, these drugs often cause minimal damage to tumors, because of MDR, and this can result in the expansion of populations of MDR tumors. Also, most chemotherapy drugs have very narrow therapeutic windows, frequently showing toxicity to healthy tissues and organs even at doses lower than required for a therapeutic effect. Therefore, there is an urgent need to devise ways to achieve high doses in tumor cells while eliminating harm to healthy tissue.
Chen concludes, “The mechanism of MDR is interesting scientifically, but also incredibly important medically. That is why we are using our bioengineering skills to develop strategies to optimize the effect of these drugs on the cancer while reducing the toxicity to the surrounding tissues, which is both a major impediment to successful treatment as well as extremely taxing for cancer patients.”
Source: National Institute of Biomedical Imaging and Bioengineering
09.09.2016