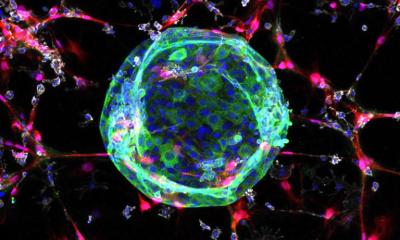
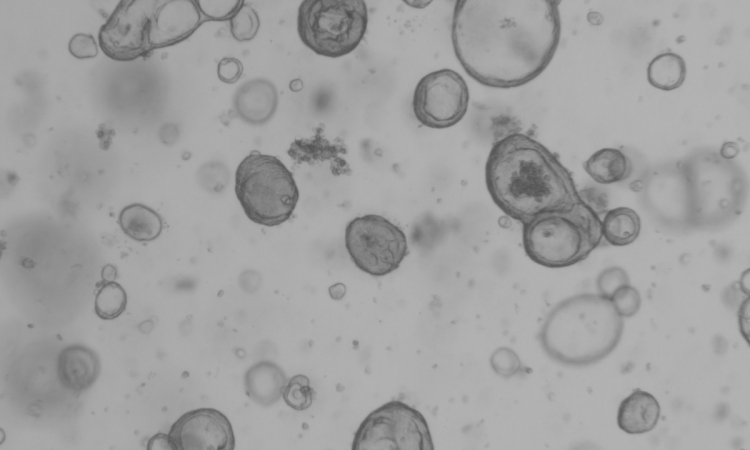
Image source: Henrik Renner, Max Planck Institute for molecular Biomedicine
Article • Modelling systems for human biology
Organoid technology: new opportunities for drug discovery
Two-dimensional (2D) cultured cell lines and animal models have been the principal research tools for the past decade. ‘However, a variety of biological processes unique to humans cannot be fully replicated in animal models or immortalized cell lines,’ said Munir Al-Zeer from the Department of Applied Biochemistry at the Technical University of Berlin, Germany.
Report: Bernard Banga
Since successfully isolating human embryonic stem cells (ESCs) and reprogramming human somatic cells into induced pluripotent stem cells (iPSCs), three-dimensional (3D) cell cultures have become very popular. Classed as one of these new structures, organoids can mimic stem cell niches more closely than 2D cell cultures, particularly gene and protein expression, metabolic function, and host-microbe interactions. ‘Organoids are capable of self-renewal, self-organisation, and exhibit organ functionality. Biologists call them organ-like aggregate cell structures,’ said Jürgen Knoblich, Scientific Director at the Institute of Molecular Biotechnology (IMBA) at the Austrian Academy of Sciences in Vienna.
35 human organoids reported for 15 organs
Organoid culture allows specific cell types to be generated that were previously unachievable using 2D cultures. To date, a total of 35 human organoid systems have been reported associated with some fifteen organs from the brain to the bladder, including the retina, the thyroid gland, blood vessels, mammary glands, and the stomach. Consequently, pancreatic, liver, and cardiac organoids have been used for disease modelling, drug efficacy testing and toxicity analysis. Brain organoids are helpful for modelling various neuropsychiatric disorders and neurodegenerative brain conditions. One recent study from the Department of Human Biology at the University of Cape Town Faculty of Health Sciences in South Africa, published in Gene Therapy, has opened up progress in understanding autism spectrum disorders. Lung organoids have been developed from various cell types such as human pluripotent stem cells (PSCs) and alveolar epithelial progenitor cells. These organoids were developed either from PSCs, or adult stem cells (AdSC) and biobanks.
Infections, respiratory diseases, cystic fibrosis, and colorectal cancer
Following the development of human stem cell-based organoids, various human diseases have been modelled using these systems. ‘Today, human brain organoids are now mainly being used to successfully model human diseases such as microcephaly, a neurological disorder seen during the Zika virus outbreak,’ said Jihoon Kim from the Institute of Molecular Biotechnology at the Austrian Academy of Sciences. Parasitic diseases such as life-threatening cryptosporium diarrhoea and bacterial infections caused by Salmonella typhi and Clostridium difficile have been successfully modelled thanks to organoid systems.
More recently in respiratory disease, lung organoids infected with SARS-CoV-2 were used to demonstrate that the expression of transmembrane serine protease 2 (TMPRSS2) is essential for priming the spike protein of angiotensin-converting enzyme 2 (ACE2) in coronavirus. ‘The high-throughput screening of lung organoids has also enabled identification of drugs that inhibit the entry of various coronaviruses into host cells,’ observed Yun Kee Jo, from the department of Biomedical Convergence Science & Technology, Kyungpook National University, Daegu, South Korea, in an article published recently in Materials Today Bio.
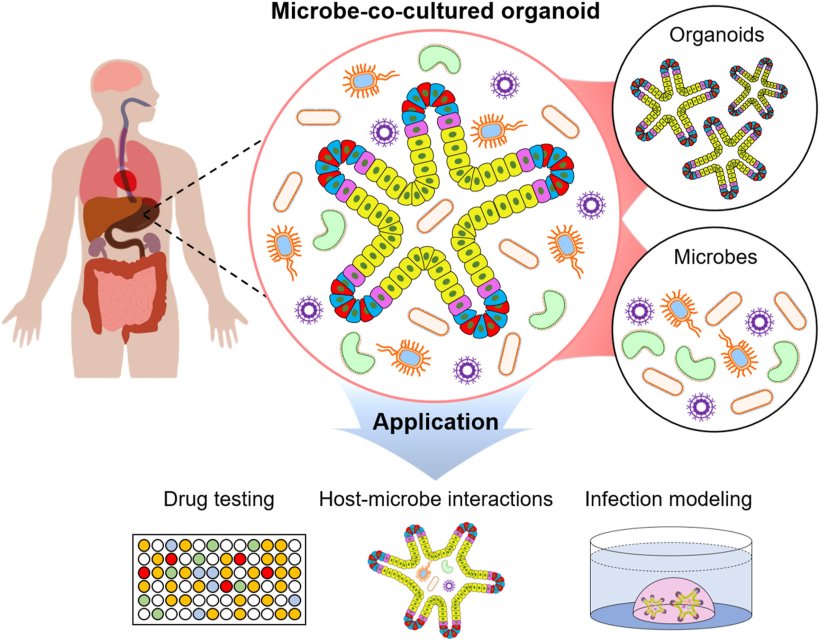
Image source: Kim et al., Materials Today Bio 2022 (CC BY 4.0)
Human organoids are becoming important resources for precision medicine They can be useful for selecting an appropriate drug for patients with genetic disorders such as cystic fibrosis, a relatively common specific genetic disorder with approximately 90,000 sufferers worldwide. Organoids can also be used as in vitro cancer models, with high morphological and molecular similarity to their origin, that can be generated from a wide variety of tumours and normal tissue. They can be used as a promising preclinical model for gastrointestinal cancer, and particularly colorectal cancer. The team from the DKFZ-Hector Cancer Institute at the University Medical Center Mannheim, Germany, collected data relating to around 5.5 million single organoids from 11 different colon cancer patients with more than 500 distinct small molecule perturbations.
Laboratory robots capable of generating up to 20,000 brain organoids a day
3D organoids are likely to become an indispensable tool for biological research, drug discovery and drug screening within a few years
Agnieszka Zuchowska
A team of scientists at the Max Planck Institute for Molecular Biomedicine in Münster, Germany, has succeeded in using human cells to produce midbrain organoids using a fully automated process. First, a strategy was devised that created mid-brain organoids from small molecule neural precursor cells which can generate all relevant mid-brain cells more quickly and with greater reproducibility than current methods. Henrik Renner’s team there then demonstrated that their automated pipetting robots could produce large quantities of organoids. This fully automated high-throughput workflow for 3D-based chemical screening in human midbrain organoids allows scientists to generate, culture and test up to 20,000 brain organoids a day. This technology has been licensed to the American biotech firm StemoniX, which has developed iPSC-based 3D micro-organ tissue constructs, disease models and advanced analytical techniques to ensure that only the safest and most effective compounds progress through the discovery pipelines of their biopharma partners.
‘3D organoids are likely to become an indispensable tool for biological research, drug discovery and drug screening within a few years,’ said Agnieszka Zuchowska, Chair of Medical Biotechnology at the Warsaw University of Technology Faculty of Chemistry in Poland. New drug development is a long (15 years) and costly process. The average cost of each new drug launched onto the pharmaceutical market is over $1 billion. According to US-based Insight Partners, the market for organoids is projected to reach $3.43 billion globally by 2027. It is expected to grow at a CAGR of 22.1% over the next five years.
Some limitations still to overcome
Although organoids are robust research tools for the study of human development and disease, there are hurdles and limitations associated with using organ-like aggregate cell structures. First, culture techniques are not standardized, hence their heterogeneous nature. Second, the absence of blood vessels impedes the maturation and function of organoids. Lastly, the lack of a native micro-environment precludes studies concerning the interaction of stem cells with their niche or immune cells.
While still in their infancy, organoids face many challenges. They merely imitate a small part of the human body, not the whole thing. Human organoids still lack communication with nearby organs or tissues and are limited to studying the reproduction of organ-specific or tissue-specific microphysiology in comparison to 2D culture and animal models. ‘There is a long way to go before implementing the first human multiorganoid model in vitro’, Zuchowska concluded.
Latest developments
The FDA announced, that this summer, it is allowed an existing drug to be repurposed based on efficacy data generated using these advanced cell models, or organoids. This represents a true breakthrough in validating the utility of these microphysiological systems and an important advance in finding treatments for rare diseases.
Orlando, Florida-based Hesperos Inc. showed that exposing its model of peripheral motor neuron conduction velocity to serum from chronic inflammatory demyelinating polyneuropathy (CIDP) patients led to increased antibody binding and activation of the complement cascade. A rare autoimmune disease that causes muscle weakness and impairs walking and hand function. CIDP is characterized by immune system hyperactivity sparked by auto antibody production, leading to peripheral nerve demyelination and reduction in nerve conduction velocity.
The data generated provided the rationale for testing Sanofi SA’s sutimlimab , a humanized monoclonal antibody used to target C1s in treating CIDP. The product gained US FDA approval under the brand name Enjaymo in February 2022 for the treatment of cold agglutinin disease, in which activation of the complement cascade leads the immune system to destroy healthy red blood cells.
Hesperos aims to replicate that success in other rare diseases for which there are no animal models. ‘There are 7,000 rare diseases and only 400 research programs looking at them, as there are no animal models. In some cases, these systems fill a void where animal models don’t exist,’ said James Hickman, CEO of Hesperos.
01.09.2022