Image source: Dr. Xiao Qin in Tape Lab at UCL
News • Patient response testing
New method predicts which cancer therapies work (and which don't)
A new technology that can study which therapies will work on patients with solid cancerous tumours has been developed by scientists at University College London (UCL).
Researchers say the tool, which can rapidly test tumorous tissue against different treatments, such as chemotherapy, immunotherapy or radiotherapy, could be used by clinicians to pinpoint the best therapy for a particular patient. Currently it is difficult for doctors to know which treatment a patient will respond to, so several different therapies may be tried before one works. The technological breakthrough, published in Nature Protocols, builds on the team’s previous work. In 2020 they developed a method that can simultaneously measure the behaviours and interactions of millions of different cells, living inside lab-grown tumours.
Our long-term vision is for the tool to be used as ‘standard’ in clinical decision-making for solid cancer treatment
Chris Tape
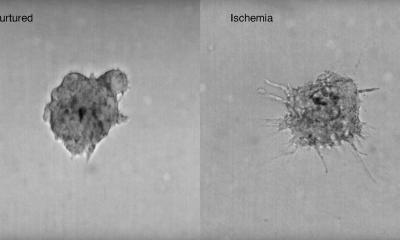
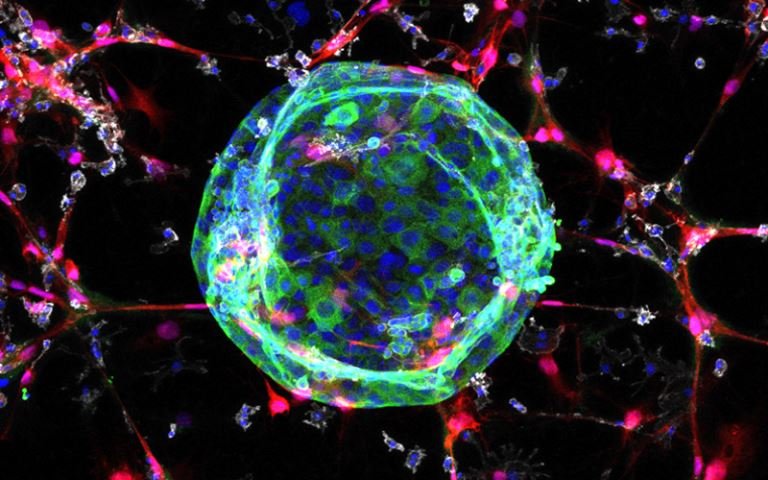
The research provided new insight into how mutated cancer cells “mimic the growth signals” normally expressed by healthy cells – which allows cancer cells to grow unchecked. In this new study they have taken patients’ cells to develop mini-tumours, known as organoids, which are grown by embedding cancer stem cells in collagen in the lab. Each mini-tumour acts as an individual patient ‘avatar’ that can be studied in the lab. The UCL team have advanced their original technology to now study hundreds and thousands of patient mini-tumours at a time. This allows researchers to trial lots of different anti-cancer drugs to explore how an individual’s tumour might respond.
Lead author Dr Chris Tape (UCL Cancer Institute) said: “The screening platform enables us to observe cancer cells, alongside the healthy immune, fibroblast and stromal cells, and see how they respond to each other – so we can model how an individual patient’s cancer behaves. By treating the mini-tumours with different kinds of cancer treatments, the screening tool also allows us to observe how both the cancer and healthy cells respond – both equally as important – and determine which treatment could work best for a patient.”
While further validation will be required via clinical trial, researchers are hopeful the new tool could transform therapy selection for people diagnosed with solid cancers including colorectal, liver, breast and brain cancers. Dr Tape added: “Our long-term vision is for the tool to be used as ‘standard’ in clinical decision-making for solid cancer treatments. A patient would have a tumour removed during surgery, the tumour cells would be sent to a laboratory and grown into an organoid avatar, which would then be tested against different therapeutic options and analysed with single-cell resolution using this new technology. The lab would then relay the findings back to the clinician treating the patient, saying this patient responds best to this drug.”
Globally researchers are now using organoids to study cancer. The 3D mini-tumours contain lots of different cells and more accurately represent a patient’s cancer, compared to more traditional research, which looks at a collection of identical cells grown in 2D. This development means cancer can be studied in more detail than ever before. However, to this point, most methods used to study mini-tumours have involved grinding up all the cells and analysing it as a mixture. This limits scientists’ ability to assess how individual cells behave or how tumours collaborate with other healthy and immune cells known to influence cancer outcomes.
To overcome this, researchers at UCL Cancer Institute, have developed a new mass spectrometry platform to measure communication signals in millions of single cells from bowel cancer mini-tumours. Specifically, researchers at UCL have developed a new technique to prepare cells for analysis on a mass spectrometer. At the subcellular level, heavy metal-tagged antibodies are attached to individual proteins (known as PTMs): these PTMs are found within all the different cell types (stem, immune, etc). The different weight of each heavy metal allows the mass spectrometer to differentiate the different proteins and analyse their behaviours and signalling activity within and between the different cell types. More than 40 types of PTM were tagged in this process, enabling researchers to build detailed ‘circuits’ that describe how the cancer cells are working.
Recommended article

Article • Clinical pathology
Focus on mass spectrometry
Mass spectrometry is pushing the boundaries of clinical pathology. Keep up-to-date with the latest research news, developments, and background information on the technique.
This pioneering tool means that, for the first time, scientists can now study how cancer cells interact with any cell type using mini-tumour models. Dr Tape said: “Up to now testing cancer treatments on individual organoids has been a bit simplistic; you would apply drugs to the organoids and see if the cells were alive or dead – so provided limited biological information. Our platform allows us to observe how and why the treatments are working, or not working; at the single cell level we can view what biological pathways are switched on or switched off, this enables us to see if cells are dying or just stop growing – a significant difference.”
Researchers improved the screening tool enabling them to test 126 organoids (i.e. patient samples) in a single screening process, an order of magnitude more than before. “It is one of the great frustrations that with common chemotherapies, some patients will respond well and others not at all,” added Dr Tape. “The idea is that we can predict that in the lab, provide an early check and balance, and hopefully find an alternative treatment to use at the outset of a patient’s treatment course.”
Source: University College London
09.09.2021