Image source/Copyright: Deutscher Zukunftspreis/Ansgar Pudenz
News • Photon counting
Major CT advancement receives FDA clearance
The U.S. Food and Drug Administration (FDA) cleared the first new major technological improvement for Computed Tomography (CT) imaging in nearly a decade.
“Computed tomography is an important medical imaging tool that can aid in diagnosing disease, trauma or abnormality; planning and guiding interventional or therapeutic procedures; and monitoring the effectiveness of certain therapies,” said Laurel Burk, Ph.D., assistant director of the Diagnostic X-ray Systems Team in the FDA’s Center for Devices and Radiological Health. “Today’s action represents the first major new technology for computed tomography imaging in nearly a decade and underscores the FDA’s efforts to encourage innovation in areas of scientific and diagnostic progress.”
Recommended article

Article • Real-time radiation plans, X-core MRI
Significant advances in medical physics
To target ionised radiation as precisely as possible, imaging a tumour is vital in radiotherapy planning. ‘Today, imaging is used increasingly during the therapy itself,’ explained Professor Mark Ladd during the 51st annual meeting of the German Society for Medical Physics (DGMP).
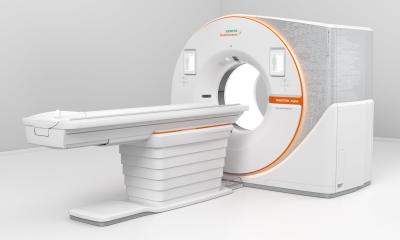
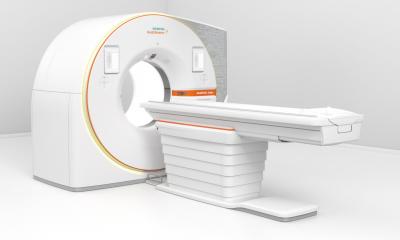
The device uses the emerging CT technology of photon-counting detectors which can measure each individual X-ray that passes through a patient's body, as opposed to current systems which use detectors that measure the total energy contained in many X-rays at once. By ‘counting’ each individual X-ray photon, more detailed information about the patient can be obtained and used to create images with less information that is not useful in the review and analysis.
The new diagnostic imaging device, called Siemens Naeotom Alpha, is designed to transform the information from X-ray photons that pass through a patient's body, and are received by a detector, into a detailed 3-dimensional image. The images delivered by the system can be used by a trained physician as an aid in diagnosis or can be used by trained staff as an aid in diagnosis, treatment preparation and radiation therapy planning.
The FDA reviewed the scanner through the 510(k) premarket clearance pathway. A 510(k) is a premarket submission made to the FDA to demonstrate that a new device is substantially equivalent to a legally marketed predicate device. The FDA granted clearance of the device to Siemens Medical Solutions Inc.
Source: U.S. Food and Drug Administration
01.10.2021