HIV
Preventing mother-to-child transmission during pregnancy
By Tamar Jehuda-Cohen PhD, Biomedical Engineering Department, Technion Institute of Technology, Haifa, Israel
HIV infection is transmitted from mother to foetus, and 28-30% of babies, born to (untreated) HIV positive mothers, are infected with HIV. Since the risk of transmission to the foetus is directly proportional to the viral levels in the mother’s blood, any reduction in viral load could improve the chance of the baby to start life without that deadly infection. This reduction can be achieved by antiretroviral drugs (ARV). However, these could have adverse effects on the foetus, especially during the first trimester.
Due to the very high exposure to the mother’s blood during labour and birth, these are the most critical stages to reduce the risk of exposure.
Based on all the above, antiretroviral (ARV) treatment protocols for HIV infected pregnant women (who are not on ARV treatment prior to pregnancy) have been developed. When possible, ARV (usually AZT) is taken from weeks 14-34, as well as during labour. Since
the exposure of the foetus is the greatest during labour and delivery, a preventive/prophylactic ARV treatment is given to the baby for the first 1-6 weeks of life. The combined mother and child ARV regime can reduce mother-to-child transmission of HIV to as low as 1-2%. Even where resources are limited, a single dose of medicine given to mother and baby can halve the risk.
The fact that the ARV regime is a short one and its effect in saving lives is so dramatic, lead to broad implementation of ARV-pregnancy-protocols globally, including the poorest and most inflicted areas, because the treatment is manageable, cost effective, and of limited duration, and, most importantly, because we are responsible for the well-being of newborns.
The key to implementing any ARV protocol is detecting HIV infected mothers. In some countries and states all pregnant women are tested for HIV (unless they opt not to). However, due to the ‘window period’ (WP = the time between infection and possible diagnosis) women who were infected recently might test negative, in spite of the infection that is risking their foetuses, and thus not be offered any ARV. This WP, which usually lasts up to three months, could be even longer in pregnant women, because the pregnancy itself is somewhat immuno-suppressive (to protect the foetus).
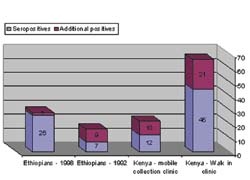
Overcoming the WP problem has been a major diagnostic concern. It has been found that specific immune suppression causes the window period. A technology, Stimmunology, has been developed, which enables detection of antibodies even during the WP (Fig 1). This is achieved by pre-incubation of the blood sample, in a SMARTube for stimulation of antibody production. In a study in Kenya, 20 pregnant women were tested for HIV with and without the SMARTube step. Eight were seropositive, yet, among the seronegative women, there were an additional five who were in the WP, and their HIV infection was detected only after pretreatment of sample with SMARTube. These women sero-converted within 3-6 months (4/5), too late for ARV to protect the babies form HIV infection. Today, the SMARTube has the CE Mark and can be used to detect HIV infected pregnant women even during the WP.
Misdiagnosis of HIV infection due to the WP contributes daily to the spread of the epidemic in the adult population. A missed infection in a pregnant mother is a missed chance to save a baby. Bridging the gap of the HIV WP in this population should be of top priority. Early and complete detection of HIV infections among future mothers is a critical key to curtailing the epidemic and saving future lives.
01.03.2008