Article • In the brain
Gadolinium deposition: A real threat or a phantom debate?
The European Medicines Agency (EMA) decided to suspend authorisation for certain linear gadolinium agents. The review by the Committee for Medicinal Products for Human Use (CHMP) states: “There is currently no evidence that gadolinium deposition in the brain has caused any harm to patients; however EMA has recommended restrictions and suspensions for some intravenous linear agents in order to prevent any risks that could potentially be associated with gadolinium brain deposition.” European Hospital talked to Dr Alexander Radbruch, radiologist at the German Cancer Research Center in Heidelberg and at University Hospital Essen, who published several articles on gadolinium deposition in the brain.
Interview: Sascha Keutel

Dr Radbruch, would you explain the decision of EMA, to suspend the authorisation of four intravenous linear gadolinium based contrast agents for most of the indications in the EU?
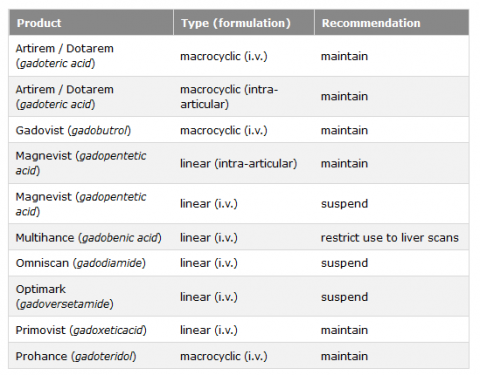
‘Generally, a comprehensive risk benefit analysis has to be performed prior to the administration of every medication. For gadolinium based contrast agents (GBCAs) this risk-benefit analysis includes - among other factors such as diagnostic efficacy and the risk of further side effects - also the potential gadolinium deposition risk. Even though we do not know if any clinical correlates exist for the gadolinium deposition, the EMA decided that it is favorable to minimize the amount gadolinium in the body. Since numerous studies provided evidence that the total amount of gadolinium in the brain can be reduced when macrocyclic GBCAs are used the EMA pursued a “precautionary approach” and concluded that the risk benefit ratio is no longer favourable for the linear GBCAs. Exceptions of this regulation are exclusively the use of gaobenate or gadoxetic acid for liver imaging and gadopentetic acid for intraarticular joint imaging.
Unlike the EU, the USA’s regulatory body, Food and Drug Administration (FDA) currently does not see a need for action. Why are their opinions so different?
‘The FDA acknowledges that linear gadolinium based contrast agents deposit more gadolinium than macrocyclics. However, they come to a different conclusion within their risk benefit analysis and it seems that they will not remove any product from the market as long as no clinical correlates of gadolinium deposition are proven.’
Does the decision to restrict authorisation without proof of damage lead to unnecessary confusion among physicians? Do you understand this growing insecurity, even patient’s fears?
‘In my opinion, the increasing uncertainty among physicians and patients as well as an unreasonable decline of GBCAs in clinically indicated situations is indeed the biggest threat in the current debate. Personally, I hope that the uncertainty will subside as time goes by. It’s important to point out that the entire debate might be a phantom debate - an issue without substance. Generally, we need to explain our patients that GBCAs are an important component of clinical routine(Article in german)and often allow life-saving diagnoses. To date, GBCAs have been applied approximately 450 million times worldwide – 50 percent of these might have been linear and despite of this enormous number, no clinical correlates of the depositions are known, yet.
However, we should not forget that it also took us almost 10 years to disentangle the correlation between gadolinium and NSF. This experience with NSF might be one of the reasons why the EMA followed its precautionary approach.'
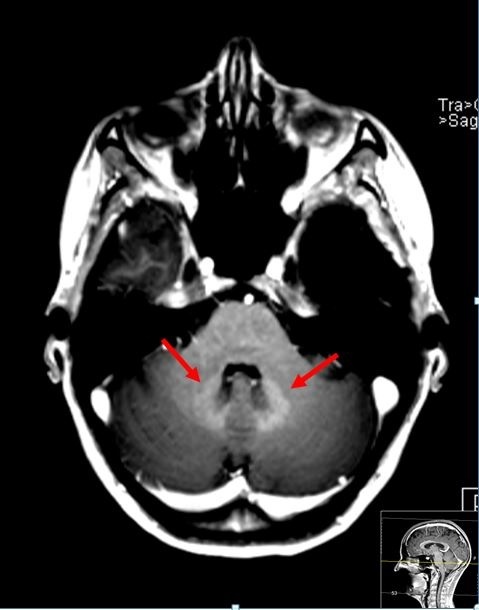
What is the current research status?
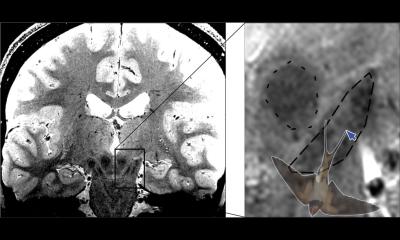
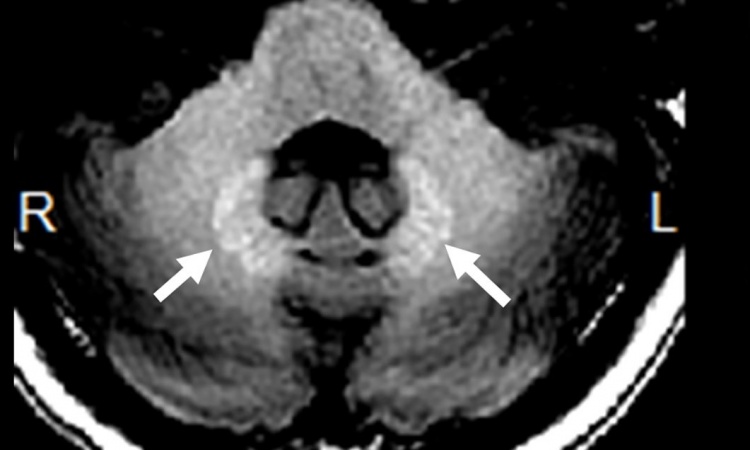
‘Animal experiments indicate that 24 hours post injection similar amounts of GBCAs – linear as well as macrocyclic – can be found, first in the cerebrospinal fluid and 24 hours later in the brain. However, four weeks after injection the total concentration of gadolinium in the brain is much higher with linear GBCAs than with macrocyclics – the latter being just slightly above the level of detection. Further animal experiments provided evidence to explain this finding: While the intact chelate is washed out over time for both - linear and macrocyclic GBCAs - the linear GBCAs partly dechelate and subsequently bind to different partners (eg macromolecules) which finally cause the hyperintensities seen on T1 weighted images. In macrocyclic GBCAs, however, no gadolinium release was detected. Consequently, the statement that all contrast agents deposit gadolinium is partly misleading. Gadolinium deposition is a process which has to be monitored over time: the intact chelate can temporarily be detected in the brain after both linear and macrocyclic GBCAs administration. However, dechelated gadolinium, which potentially stays in the brain, is found only after use of linear GBCAs.’
Are there potential alternatives to gadolinium-containing agents?
‘Currently, a lot of research on contrast-free MRI techniques is being conducted. At the German Cancer Research Centre we recently showed that sugar might potentially be used as contrast agent for brain tumour patients. Moreover, I’m sure, going toward diffusion-weighted imaging, which does not require contrast agents at all, will play a crucial role. However, I don’t see any technique that, in the short run, might replace GBCAs.
Could the debate lead to an overall ban?
‘I’m sure there won’t be an overall ban. A ban would be absurd in view of the immense clinical benefit of GBCAs. I do hope that, in Europe, suspending the authorisation for linear GBCAs will calm the waves. Nobody can tell where the regulatory debate in the USA is heading. At the end of the day, I guess, markets will create facts. Look at paediatrics: Over the past two years, this field has witnessed a major shift in the USA and today 95 percent of GBCAs used are macrocyclic. Such developments might make regulatory decision superfluous.’
Profile:
PD Dr. Alexander Radbruch is a radiologist at the German Cancer Research Center in Heidelberg and at the University Hospital Essen. He has published numerous papers on gadolinium retention and in 2015 showed for the first time that differences in gadolinium retention in the brain exist between macrocyclic and linear gadolinium-containing contrast agents. In 2017, the radiologist was chosen by colleagues in the category "Radiological Research" as one of the world's 15 most influential people.
27.02.2018