Article • Contrast agents
Reason must prevail in debates on GCCAs use
Radiologists must ensure precise scientific data and radiology-based evidence are used to regulate the use of Gadolinium Containing Contrast Agents (GCCAs), a Spanish leading radiologist explained in closed-door leadership meeting earlier this year in Barcelona.
Report: Mélisande Rouger

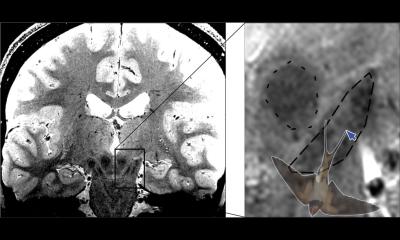
Findings showing GCCAs deposits in different parts of the body have triggered a profusion of publications and decisions that now directly affect radiology practice. The good news is that radiologists can use their abilities to bring science back into the discussion, according to Luis Martí-Bonmatí, Director of the Medical Imaging Department and Biomedical Imaging Research Group at La Fe University Hospital in Valencia, who addressed a panel from the academy and the industry. ‘There’s been a real tsunami; large quantities of diverse information and opinions that have pushed the authorities to make decisions, which affect our daily practice but are based on neither accurate nor precise data,’ he said.
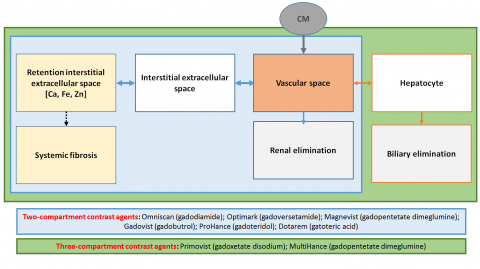
Radiologists now understand the full extent of the deluge. ‘We may not have valued these decisions properly at the time. Usually we are not involved in these discussions, so it’s not easy to form an initial opinion,’ Martí-Bonmatí explained. In the past three years, concern has grown about gadolinium deposits in the brain of patients undergoing GBCA-enhanced MR examinations. In 2017, the Pharmaco-vigilance Risk Assessment Committee (PRAC) of the European Medicines Agency (EMA) recommended suspension of some GCCAs marketing authorisations based on linear chelators, due to the potential risk of gadolinium retention in the human body; the recommendation was further extended by EMA’s Committee for Medicinal Products for Human Use. As a result, four linear GCCAs are no longer marketed in the EU (Omniscan, Magnevist, Optimark and MultiHance).
Clear clinical evidence is missing
However, even if long-term safety of GCCAs remains unclear, current scientific evidence for gadolinium retention has several methodological limitations, most experts feel. ‘No clear clinical evidence exists indicating that gadolinium retention causes neurotoxicity. We need this evidence to formulate a hypothesis,’ Martí-Bonmatí said. ‘If we don’t use that approach, hypotheses simply do not stand.’
In Spain, the Medicine and Medical Devices Agency is following the EMA recommendation, arguing that clinical benefits do not overcome potential risks. But it still enables the use of Primovist and MultiHance for hepatic studies, an exemption Martí-Bonmatí finds intriguing. ‘That’s a backroom door! If risks do exist, then they should be relevant in any application and in all contrast media,’ he said. ‘Even more importantly,’ he added, ‘we know today that all GCCAs are retained in tracer quantities in different compartments, but without any histological toxicity or clinical sign.’
Debates on potential risks of cutting-edge technology had spurred in the past over the use of radiofrequency and, more recently, magnetic resonance imaging, as an EU directive planned to limit the exposure of workers to magnetic resonance. Eventually, the proposal was withdrawn.
‘This draft would have significantly restricted the use of a technique that has showed its benefits in millions of patients. GCCAs have been administered more than 300 million times, with a very low frequency of acute adverse events. People tend to overestimate the occurrence of very unlikely events and magnify them in the decisions they make. And generally, people lack abilities to correctly interpret statistical probabilities,’ Martí-Bonmatí pointed out.
Precise data to evaluate risks is needed
Radiologists can help evaluate reliability and relevance of risk management investigation using their scientific reasoning. ‘What the world needs now are cool, reason-based facts to evaluate risks appropriately. We need to use the scientific method, i.e. to carry out consequent observation using experimentation, measurements, formulation, analysis and hypothesis renovation, to generate evidence,’ he said.
However, as they offer their expertise to help advance knowledge, radiologists must demand strong data in return.
‘Us radiologists should have a clear idea on how to evaluate data precision, and trust our critical and criteria appraisal for the patients benefit. Each time an institution, academy, committee, person or group approaches us with recommendations on how to act in our clinical practice, we should ask which data and evidence they base their opinion on, to be able to use our reasoning,’ he said.
Bias awareness
Not only are most investigation results false but also, when they are accurate, they are useless
Luis Martí-Bonmatí
When faced with the vast amount of studies on the potential risk of GCCAs, radiologists must be aware of bias inherent to scientific investigation. In radiology, bias can originate from samples, tools and data used in measurements and analyses.
But even when trials are carried out properly, results often prove useless, Martí-Bonmatí explained. ‘Not only are most investigation results false but also, when they are accurate, they are useless. That’s because, deep down, we base our evaluation on statistical probability and we can’t or don’t know how to, or haven’t properly extrapolated small samples’ results from the general population. And,’ he added, ‘we haven’t been able to properly manage these small probabilities.’
To this day the only known adverse effect linked with GCCAs use is nephrogenic systemic fibrosis, which had a very low incidence; the risk has almost disappeared since doses are now tailored to each patient. Recent research even suggests it has no effect in patients undergoing dialysis or with chronic renal disease (https://www.ncbi.nlm.nih.gov/pubmed/28731375)
Profile:
Luis Martí-Bonmatí MD PhD is Director of the Medical Imaging Department and Biomedical Imaging Research Group (GIBI230) at La Fe Polytechnics and University Hospital and Health Research Institute in Valencia, Spain. He is also professor of radiology at Valencia University
ECR 2018
Wednesday 28, February
16:00-17:30 Room G
Special Focus Session, Level II
SF 4 Gadolinium deposition: is it harmful?
27.02.2018