PET study
Functional interplay between two transporters at blood-brain barrier
A team of researchers at the MedUni Vienna has, in collaboration with the AIT Austrian Institute of Technology, developed two new PET tracers that allow the activity of drug transporters at the blood-brain barrier to be measured. The studywas able to demonstrate that people with a genetic polymorphism in a transporter gene have lower transporter activity at the blood-brain barrier, which can lead to an increased risk of adverse drug interactions in the brain.
The blood-brain barrier is a barrier between blood and the brain which protects the brain from unknown substances. A key part of this protective function is carried out by ABC transporters, which are able to prevent small molecules from crossing the blood-brain barrier. These transporters, however, also prevent many drugs from entering the brain and so being therapeutically active there.
In studies involving animal models, it was possible to show that the two transporters ABCB1 (P-glycoprotein) and ABCG2 (breast cancer resistance protein) work together at the blood-brain barrier and prevent dual ABCB1/ABCG2 substrates such as tyrosine kinase inhibitors (e.g. gefitinib, erlotinib, lapatinib) from entering the brain. If the activity of just one of the two transporters is inhibited, the other transporter continues to prevent the passage through the blood-brain barrier. This sophisticated protection mechanism means that dual ABCB1/ABCG2 substrates can only reach the brain if both transporters are inactive.
Functional interplay in animal models also demonstrated in humans
Until now, it was not known whether this functional interplay between ABCB1 and ABCG2 also occurs at the human blood-brain barrier. A team of researchers from the MedUni Vienna led by Oliver Langer and Martin Bauer from the Department of Clinical Pharmacology (interim head Markus Zeitlinger) has worked closely for many years with the Division of Nuclear Medicine (headed by Marcus Hacker) at the Department of Biomedical Imaging und Image-guided Therapy (headed by Christian Herold), Rudolf Karch from the Center for Medical Statistics, Informatics and Intelligent Systems (headed by Wolfgang Schreiner) and the AIT Austrian Institute of Technology to develop two new radiotracers for positron emission tomography (PET) ([11C]tariquidar, [11C]elacridar). These two radiotracers have allowed the scientists for the first time to measure the activity of ABCB1 and ABCG2 at the blood-brain barrier in a non-invasive manner.
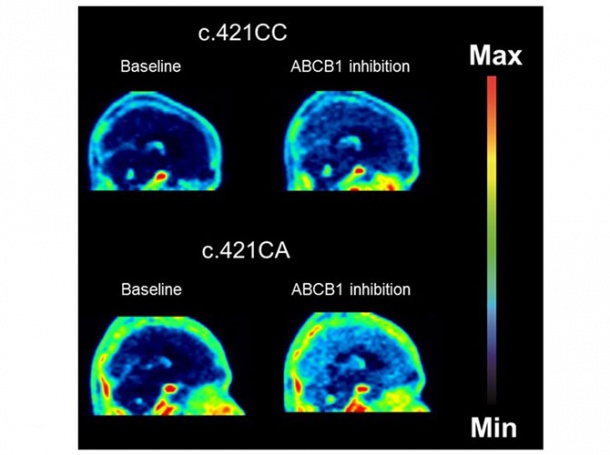
The study was performed in healthy volunteers to demonstrate that the sole inhibition of ABCB1 leads to no notable increase in the brain distribution of dual ABCB1/ABCG2 substrates, which for the first time confirms a functional interplay between ABCB1 and ABCG2 at the human blood-brain barrier.
Individual gene variations make the blood-brain barrier permeable
In people with a genetic polymorphism of the ABCG2 gene (c.421C>A) – which would be around 20 per cent of the population – there was, by contrast, a significant increase in brain distribution of dual ABCB1/ABCG2 substrates when ABCB1 was inhibited.
This shows for the first time that the activity of ABCG2 at the blood-brain barrier is reduced in carriers of the ABCG2 polymorphism. As a result, drugs that are transported by ABCG2 can enter the brain of carriers of the polymorphism better than those who are not carriers. Conversely, the polymorphism can also increase the risk for transport-mediated drug-drug interactions, which in certain cases could result in adverse side effects of drugs in the brain.
This study makes an important contribution to personalised medicine by outlining for the first time the effects of genetic polymorphisms and targeted transporter blockade on concentrations of drugs in the central nervous system.
Source: MedUni Vienna
06.04.2016