© Image available from: https://www.researchgate.net/figure/Lightning-black-and-Cb-TRAM-grey-objects-a-Pixel-based-analysis-the-pixels_fig3_258748304 [accessed 11 Apr, 2019]
Article • Revolution through AI
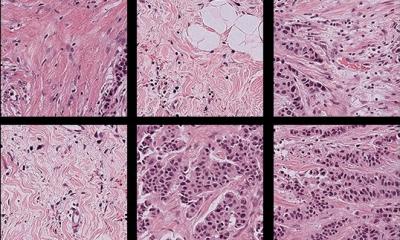
Pixel analysis: the new era of digital pathology
As reporting workload for pathology departments continues to rise rapidly, artificial Intelligence solutions are set to play an increasing role in daily practice.
Report: Mark Nicholls
What is it that AI can do that the pathologist can’t? These are areas in which we expect the next major advances to be made
David Snead
In many pathology departments the annual number of cases has risen by around 2-4% but the slides produced has doubled in the last decade. Histopathologist Professor David Snead identified this phenomenon within his own centre in the United Kingdom and outlined a similar scenario during his presentation – ‘Disease processes by pixel analysis: the new era of digital pathology’ – at the Digital Pathology and Artificial Intelligence Congress held in London in December. ‘I see this as the future of digital pathology,’ he said, when he outlined how deep learning and artificial intelligence (AI) is on the way to revolutionising pathology practice. ‘As a practising histopathologist, the key reporting workload is going up; we are producing double the numbers of slides from the same number of cases and we need help as this avalanche of work continues. We need to prepare for having the pathology workload aided by computer, with image analysis taken over by the deep learning approach.’

Digital whole slide imaging (WSI) is allowing pathologists to use computer workstations for routine diagnostic work, facilitating the adoption of algorithms to assist the diagnostic process. This, he said, has coincided with development of convoluted neural networks and machine learning, delivering remarkable advances in AI; a new era for histopathology beckons he believes, in which ‘widespread adoption of algorithms to improve and standardise the reporting of diagnostic samples is anticipated.’ His presentation highlighted AI development applied to digital pathology to date, and referred to some recent studies for insights into likely developments in this field.
Snead, Consultant Histopathologist and Clinical Service Lead for Coventry and Warwickshire Pathology Services, outlined the benefits and scope of AI in cellular pathology in terms of automation; biomarker assessment; cancer detection; cancer grading; prognostic and predictive tools, and research. ‘Some aspects of the workload, particularly the screening of slides for regions of interest, will become automated, with widespread adoption of algorithms scanning slides ahead of the pathologists first review, enabling direction of attention to areas of importance,’ he predicted.
Breast cancer already features highly in the literature predominantly for image analysis of biomarkers, Snead added, and pointed to an example of Her2 scanning in a man versus machine context, in which the machine with digital image analysis outperformed manual biomarkers in breast cancer assessment.
The Camelyon 16 challenge showed that the best algorithms perform at least as well as a pathologist, he added. Importantly, Snead noted that, when pathologists were given unlimited time to analyse an image, they performed well. However when placed in a scenario of the time-constrained real-world environment of a clinical setting, they performed worse than the computer, highlighting the benefits of the pathologist and AI working in tandem in disease processes by pixel analysis. ‘When you combine the pathologist and the algorithm you get better overall results. So much for improving what pathologists already deliver – what is it that AI can do that the pathologist can’t? These are areas in which we expect the next major advances to be made.’
Such solutions will only get into the clinic if we can validate these tools and get them approved through our regulatory framework
David Snead
An important area, said Snead pointed out, is analysis of the interaction between tumour cells and their microenvironment. With surgically resected lung cancer the patient wants to know how they are going to do, he explained, while the clinician wants to know if they need further treatment. AI has benefits in tumour cell morphometrics to predict survival in lung adenocarcinoma. Similar work is being conducted for prostate cancer and breast carcinoma, he said. In several tests/challenges Snead acknowledged how the computer is at least as good as the pathologist and sometimes probably better, e.g. to predict which lung cancers harbour mutations in the EGFR gene.
The UK government is funding five centres to advance AI in image diagnostics. Digital pathology is central to three of these centres including the Coventry PathLAKE centre, of which Snead is director. This initiative is designed to connect the UK’s excellence in AI, its strength in real world NHS clinical data and the innovative SME start biotech sector, to encourage innovative ideas and solutions. However, he warned, ‘Such solutions will only get into the clinic if we can validate these tools and get them approved through our regulatory framework.’ He remains confident that this can be achieved by working with regulators and helping to align processes with the FDA, and across the EU.
Digital image analytics is moving into practice, he said, but there are many challenges that remain including interoperability across platforms, variability of sections and of staining, quality assurance of performance, implementation controls, and accreditation. A key advance would be for the elements to come together to provide algorithms that are truly interoperable and perform on different platforms, he said. ‘Digital pathology is progressing without these algorithms at the moment, but adoption will be speeded up when really useful algorithms come along.’
Profile:
NHS consultant pathologist Professor David Snead works at the University Hospital of Coventry and Warwickshire, where he is clinical lead for Cellular Pathology at Coventry and Warwickshire Pathology Services. Having led the implementation of digital pathology at the centre, his main research interests lie in extending the capability of digital pathology for routine diagnostic use and the advancement and deployment of computer assisted diagnostic algorithms.
24.06.2019