Article • Digital PET imaging
Digital Photon Counting improves diagnostic accuracy
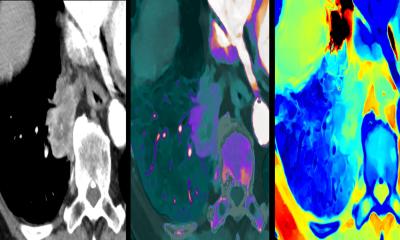
Built as the first commercially available scanner to deliver truly digital PET, the Vereos PET/CT, from Philips, offers revolutionary Digital Photon Counting technology.
Interview: Daniela Zimmermann

The science behind this scanner evolution is ‘quite complicated’, agrees Piotr Maniawski, Director of Clinical Science Nuclear Medicine at Philips Healthcare, yet the improved performance is significant, particularly when compared with an analogue system. That performance includes a reduction in scan time, lower dose, improved diagnostic accuracy and better detection of small lesions as well as applicability across oncology, cardiology and neurology, for example in dementia assessment. Maniawski outlined how proprietary Digital Photon Counting (DPC) technology sits at the core of the new Philips PET system, and was developed to overcome the limitations of conventional photomultiplier technology. With PET, scintillating crystals are used to collect high-energy photons and convert them to visible light, which is then picked up by a light sensor, and the output constructs the resulting image. With DPC technology, light is counted as individual single photons.
‘This is very technical talk,’ Maniawski concedes, ‘but what it means is that we have far more precise information about the characteristics of that light signal. We have a better way of determining when the signal was detected and also more precise localisation of that signal. The primary benefit of the improved resolution is the detectability of smaller lesions and that eventually translates into more acute diagnostic accuracy.’
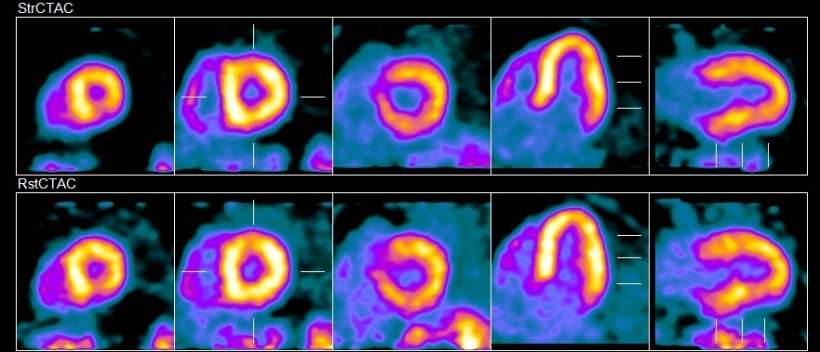
Additional to DPC, other advances making digital PET possible are 1:1 coupling between the scintillator and the light-sensing element and faster Time-of-Flight (TOF) technology. Philips’ DPC technology was developed to overcome the limitations of conventional photomultiplier technology and the 1:1 coupling and enhanced TOF allow the Vereos system to offer approximately double the volumetric resolution, sensitivity gain, and accuracy of a comparable analogue system. Maniawski emphasised how better images benefit patient management with the ability to see disease that has traditionally been difficult to image or the recurrence of disease. ‘This can change patient management if, for example, there are extra lymph nodes that were not seen before. Significant patient decisions are made on the accuracy of the detectability of the disease.’
PET tracers are enhancing personalised medicine in, for example, the area of immunotherapy and marking antibodies with PET imaging isotopes. ‘These antibodies will go only to places where they are sent; that’s a very targeted, personalised precise therapy. However, before therapy, we can image to make sure the patient receiving this immunotherapy is going to respond,’ he said, adding that the latest clinical evidence for cancers that utilise glucose for growth is that PET and PET/CT is the most accurate staging modality. With immuno-PET developed to help guide immunotherapy, researchers investigate if a therapy works in tumour response. ‘Here again digital PET has potential, because it’s much more quantitative, more reliable in absolute uptake. We can do studies and track if a patient is responding in the way we anticipate,’ Maniawski explained.
A number of academic sites are currently using Vereos PET/CT for high-end research and have developed their own radio tracers to work with Philips, using those tracers for investigational and clinical applications. Other areas where Vereos is applicable, in addition to oncology, are cardiology and neurology, such in diagnosis of different types of dementia, where various tracers are used, such as amyloid tracers of amyloid plaque, and tau (an antibody that expresses in dementia). ‘What you want in a PET scan for dementia is high resolution,’ Maniawski pointed out, ‘because we are looking at very small changes and we want to detect these changes before clinical symptoms of cognitive dementia show up. ’Another key area of evaluation from Vereos is in coronary artery disease (CAD) with heart scans conducted at peak exercise and at rest to assess the supply of blood in the myocardium. An issue with conventional PET myocardial perfusion imaging is that, in patients with multi-vessel disease, it cannot fully distinguish where the coronary arteries are diseased. ‘However, if we are able to characterise the absolute flow in millilitres per minute per gram of tissue, we can then see in absolute terms if the flow is normal or not – even in patients with multi-vessel disease – with new PET/CT scanners,’ Maniawski said. This is where the 1:1 coupling offers a critical benefit, because it allows more accurate quantification of the flow.
Artificial Intelligence (AI) – or adaptive intelligence as Philips prefers to call it – also undergoes significant developments with PET, notably for example with adaptive protocols that are specific to patients. ‘Patient image quality suffers with larger patients,’ Maniawski observed, ‘but the system can adapt the protocol either through automisation or reconstruction.’
Profile:
Piotr Maniawski is a clinical physicist and Director of clinical science for nuclear medicine and advanced molecular imaging at Philips Healthcare. He worked in a multitude of positions, including as radiation safety officer in Zabrze, Poland, as research associate at Yale University and as software engineer in Cleveland. The focus of his work is in nuclear imaging, especially PET/CT, for which he develops clinical protocols and quality control tools.
25.11.2018
- AI (824)
- cardiology (778)
- diagnostics (569)
- imaging (1640)
- neurology (613)
- nuclear medicine (127)
- oncology (463)
- patient management (287)
- PET/CT (185)