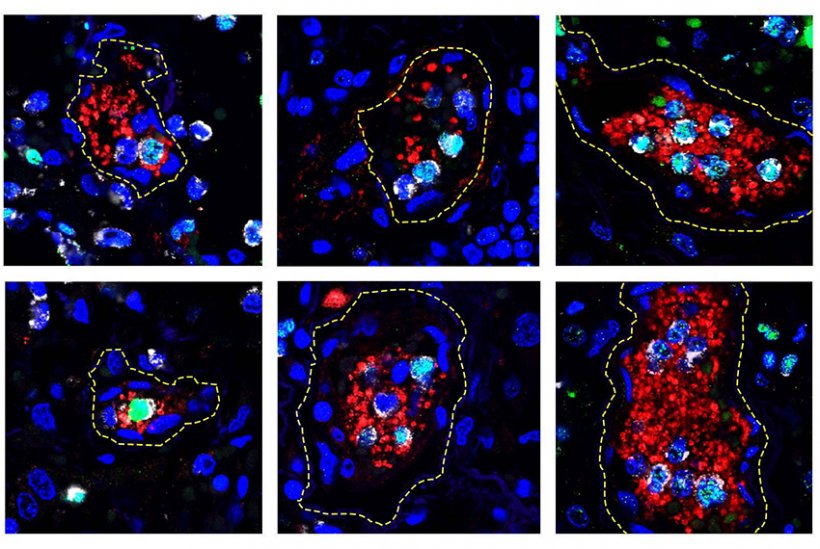
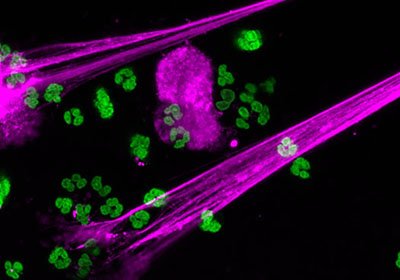
Credit: Xue-Yan He and David Ng, Egeblad Lab/Cold Spring Harbor Laboratory.
News • NET-like
Clues to COVID-19 complications come from inflammatory response
An overactive defense response may lead to increased blood clotting, disease severity, and death from COVID-19. A phenomenon called NETosis—in which infection-fighting cells emit a web-like substance to trap invading viruses—is part of an immune response that becomes increasingly hyperactive in people on ventilators and people who die from the disease.
A team led by University of Utah Health and PEEL Therapeutics, in collaboration with Cold Spring Harbor Laboratory and Weill Cornell Medicine, report the findings in a new study published in the journal, Blood.
“This study tells us about a potential mechanism for lung injury in COVID-19 that had not previously been recognized as a possible target for treatment,” says Elizabeth Middleton, M.D., the study’s first author and a critical care specialist at U of U Health.
The investigation also reports that a naturally occurring protein—originally found in umbilical cord blood—quiets this NET immune response in laboratory experiments, potentially opening new avenues for treatment.
It is estimated that up to 10% of people with COVID-19 become critically ill with respiratory distress. Causes of lung damage are a subject of intense investigation, and increasing evidence demonstrates that increased blood clotting may lead to complications caused by the disease.
Middleton, U of U Health physician-scientists and co-senior authors Christian Con Yost, M.D. and Joshua Schiffman, M.D., and colleagues, took a closer look to see if a specific immune response, called NETosis, could be involved.
As part of an immune response, white blood cells release web-like Neutrophil Extracellular Traps (NETs) to capture and kill pathogens. While typically beneficial, Yost had previously shown that overactive NETs exacerbate certain illnesses. In conditions such as overwhelming infection, NETs can clog blood vessels and lead to inflammatory tissue damage.
To determine whether NETs could be responsible for complications seen in COVID-19, the team examined plasma from 33 patients, along with tracheal aspirates from the lungs. They found that NET activity correlated with disease severity.
Patients on life support and those who died from COVID-19 had significantly more signs of NET activation than patients who were not as sick or who went on to recover. The NET immune response was lower still in healthy people. NET levels also tracked with a marker for blood-oxygen levels, an independent indicator of disease severity.
Similarly, plasma from sick patients was primed to launch the NET response. When examined in laboratory experiments, plasma from COVID-19 patients triggered white blood cells from healthy patients to shoot out 50 times as many NETs as cells exposed to plasma from otherwise healthy adults. “This study may tell us that NET levels in the blood could potentially help predict disease severity and mortality in COVID-19,” says Yost, a physician-scientist at U of U Health. “Additional information is urgently needed in this pandemic regarding how to know which patient will fare better or worse.” Larger studies will need to be done to determine whether NETs could become a biomarker for COVID-19 severity. “Importantly, we think exaggerated NETs could be a cause of morbidity and mortality in COVID-19,” Yost says.
In support of the idea, collaborators at Cold Spring Harbor Laboratory showed that blood vessels in the lungs of deceased COVID-19 patients were dotted with clumps of NET-producing cells and a critical type of blood cell for clotting, the platelets. Another recent study from U of U Health showed that platelets become hyperactive during the disease. Investigations are now underway to determine whether NETs and platelets increase the risk for blood clotting and other clinical manifestations of COVID-19.
“In COVID-19, thrombosis is a major cause of death. So, our findings tell us that we should focus on understanding more about NETs’ role in clotting in COVID-19,” Mikala Egeblad, Ph.D., a cancer researcher from Cold Spring Harbor Laboratory, says. “Thrombosis is also a major cause of death in late stage cancer, where there also can be elevated NETs in the blood. Therefore, I think that what we learn from COVID-19 will help us with other diseases, including cancer.”
Additional laboratory experiments showed that a small protein found in umbilical cord blood of newborn babies, called neonatal NET Inhibitory Factor (nNIF), quiets the hyperactive NET response in white blood cells treated with COVID-19 patient plasma. This peptide is thought to protect babies from harmful inflammation early in life, explains Schiffman, CEO of PEEL Therapeutics. His company is now evaluating whether the protein could become the basis for a clinical treatment.
“Newborns babies have a natural therapeutic in their blood to protect against these same inflammatory events that we think could be killing COVID-19 patients,” Schiffman says. “This targeted approach to stopping NETs may be more effective with less side effects than some other drugs being tested now in COVID-19 patients that block the entire immune system.”
Source: University of Utah Health
02.07.2020