Credit: Wyss Institute at Harvard University
News • Medication testing
'Airway-on-a-chip' to find new Covid-19 drugs
A collaboration spanning four research labs and hundreds of miles has used the organ-on-a-chip (Organ Chip) technology from the Wyss institute at Harvard Univesity to identify the antimalarial drug amodiaquine as a potent inhibitor of infection with SARS-CoV-2, the virus that causes Covid-19.
The Organ Chip-based drug testing ecosystem established by the collaboration greatly streamlines the process of evaluating the safety and efficacy of existing drugs for new medical applications, and provides a proof-of-concept for the use of Organ Chips to rapidly repurpose existing drugs for new medical applications, including future pandemics. The research is reported in Nature Biomedical Engineering.

Image source: Stephanie Mitchell/Harvard file photo
While many groups around the world have been testing existing drugs for efficacy against Covid-19 using cultured cells, it is well known that cells grown in a dish do not behave like the cells in a living human body, and many drugs that appear effective in lab studies do not work in patients. The Wyss team examined eight existing drugs, including hydroxychloroquine and chloroquine, that they and others had found were active against SARS-CoV-2 in conventional cell culture assays.
When tested in their more sophisticated microfluidic Lung Airway Chip, which had been infected with a pseudotyped SARS-CoV-2 virus, they found that most of these drugs, including hydroxychloroquine and chloroquine, were not effective. However, another antimalarial drug, amodiaquine, was highly effective at preventing viral entry. These results were then validated in cultured cells and in a small animal model of Covid-19 using infectious SARS-CoV-2 virus. Amodiaquine is now in clinical trials for Covid-19 at multiple sites in Africa, where this drug is inexpensive and widely available. “The speed with which this team assembled, pivoted to Covid-19, and produced clinically significant results is astonishing,” said senior author and Wyss Institute Founding Director Don Ingber. “We started testing these compounds in February 2020, had data by March, and published a preprint in April. Thanks to the openness and collaboration that the pandemic has sparked within the scientific community, our lead drug is now being tested in humans. It’s a powerful testament to Organ Chips’ ability to accelerate preclinical testing.”
In the early months of the Covid-19 pandemic when little was known about the novel SARS-CoV-2 virus, efforts were made around the globe to identify existing drugs that could be repurposed to treat patients who were falling ill. While early data performed on cells grown in lab dishes seemed to suggest that the antimalarial drugs chloroquine and hydroxychloroquine could treat the disease, later studies showed that they aren’t active against SARS-CoV-2 in animals or patients, and the quest for an effective oral therapeutic that can both treat and prevent Covid-19 continues. Fortunately, the Wyss Institute had a ready-made solution to that problem. In a move that today seems prescient, over three years ago the Defense Advanced Research Projects Agency (DARPA) and National Institutes of Health (NIH) awarded funding to Ingber’s team to explore whether its human Organ Chip microfluidic culture technology, which faithfully mimics the function of human organs in vitro, could be used to confront potential biothreat challenges including pandemic respiratory viruses.
Recommended article
News • Coronavirus genome folding
Researchers prepare for “SARS-CoV-3”
For the first time, an international research alliance has observed the RNA folding structures of the SARS-CoV2 genome with which the virus controls the infection process. This could not only lay the foundation for the targeted development of novel drugs for treating Covid-19, but also for occurrences of infection with new corona viruses that may develop in the future.
Two years into the project, the team was making steady progress using its lung Airway Chip to study drugs that could be repurposed to treat influenza virus infections. Then, in January 2020, first authors Longlong Si and Haiqing Bai heard about cases of what was being called a novel viral pneumonia in China. “That caught a lot of scientists’ attention, because any new virus could become a global threat given how easily infections spread in today’s era of widespread international travel. We closely followed the updates because we thought that our Airway Chip model could provide an important tool for studying this virus,” said Si, a Wyss technology development fellow and co-lead author. Once it became clear that people were falling ill due to the mysterious Covid-19 and not pneumonia, the team quickly shifted its focus to the novel SARS-CoV-2 virus.
We designed a SARS-CoV-2 pseudovirus that expresses the SARS-CoV-2 spike protein, so that we could identify drugs that interfere with the spike protein’s ability to bind to human lung cells’ ACE2 receptors
Haiqing Bai
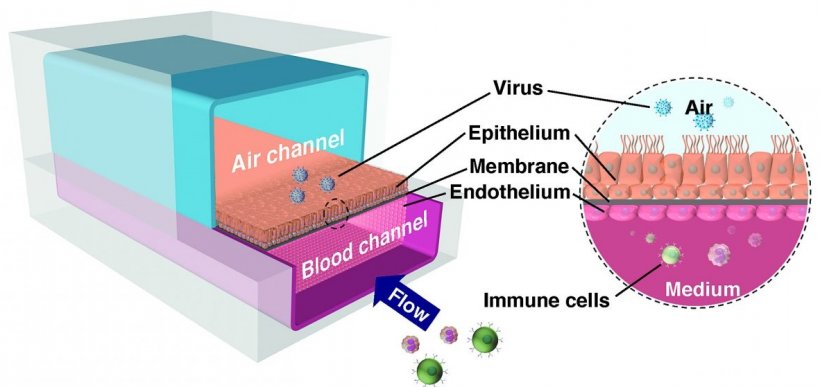
The human Airway Chip that the Wyss team developed for these studies is a microfluidic device about the size of a USB memory stick that contains two parallel channels separated by a porous membrane. Human lung airway cells are grown in one channel that is perfused with air, while human blood vessel cells are grown in the other channel, which is perfused with liquid culture medium to mimic blood flow. Cells grown in this device naturally differentiate into multiple airway-specific cell types in proportions that are similar to those in the human airway, and develop traits observed in living lungs such as cilia and the ability to produce and move mucus. Airway Chip cells also have higher levels of angiotensin-converting enzyme-2 (ACE2) receptor protein, which plays a central role in lung physiology and is used by SARS-CoV-2 to infect cells. “Our biggest challenge in shifting our focus to SARS-CoV-2 was that we don’t have lab facilities with the necessary infrastructure to safely study dangerous pathogens. To get around that problem, we designed a SARS-CoV-2 pseudovirus that expresses the SARS-CoV-2 spike protein, so that we could identify drugs that interfere with the spike protein’s ability to bind to human lung cells’ ACE2 receptors,” said Bai, who is a postdoctoral fellow at the Wyss Institute and co-lead author. “A secondary goal was to demonstrate that these types of studies could be carried out by other Organ Chip researchers who similarly have this technology, but lack access to lab facilities required to study highly infectious viruses.”
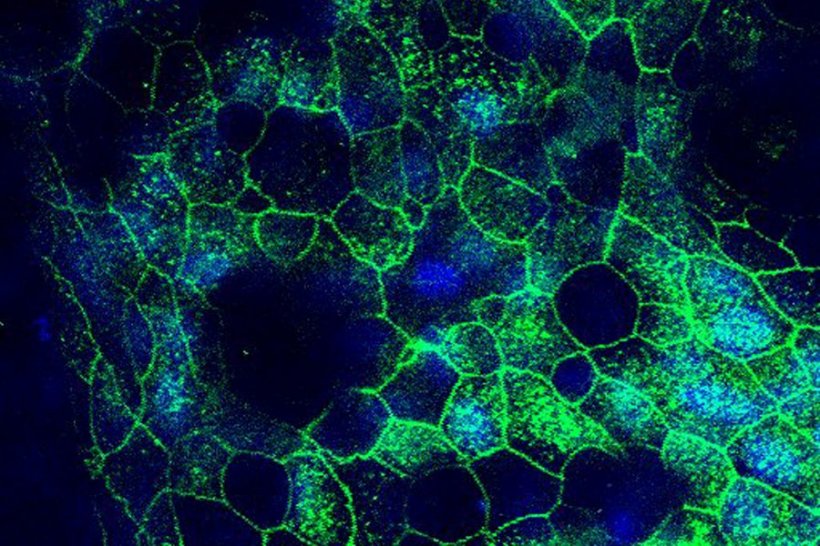
Credit: Wyss Institute at Harvard University
Armed with the pseudovirus that allowed them to study SARS-CoV-2 infection, the team first perfused the Airway Chips’ blood vessel channel with several approved drugs, including amodiaquine, toremifene, clomiphene, chloroquine, hydroxychloroquine, arbidol, verapamil, and amiodarone, all of which have exhibited activity against other related viruses in previous studies. However, in contrast to static culture studies, they were able to perfuse the drug through the channels of the chip using a clinically relevant dose to mimic how the drug would be distributed to tissues in our bodies. After 24 hours they introduced SARS-CoV-2 pseudovirus into the Airway Chips’ air channel to mimic infection by airborne viruses, like that in a cough or sneeze. Only three of these drugs ⎯ amodiaquine, toremifene, and clomiphene ⎯ significantly prevented viral entry without producing cell damage in the Airway Chips. The most potent drug, amodiaquine, reduced infection by about 60 percent. The team also performed spectrometry measurements with the assistance of Steve Gygi’s group at Harvard Medical School to assess how the drugs impacted the airway cells. These studies revealed that amodiaquine produced distinct and broader protein changes than the other antimalarial drugs.
The researchers had a lead drug candidate.
Despite the promise of amodiaquine, the team still needed to demonstrate that it worked against the real infectious SARS-CoV-2 virus. With the help of a new Covid-19-focused grant from DARPA, Ingber teamed up with Matthew Frieman at the University of Maryland School of Medicin and Benjamin tenOever at the Icahn School of Medicine at Mount Sinai, both of whom already had biosafety labs set up to study infectious pathogens. This collaboration created a drug discovery ecosystem that combines the human emulation capability of the Wyss Institute’s Organ Chips with Frieman’s and tenOever’s expertise in the interactions between viruses and their host cells. The Frieman lab tested amodiaquine and its active metabolite, desethylamodiaquine, against native SARS-CoV-2 via high-throughput assays in cells in vitro, and confirmed that the drug inhibited viral infection.
While we’re proud of what we’ve accomplished so far for Covid-19, we’re also looking forward to studying additional virus-host dynamics using the Organ Chips in the hopes that we’ll be able to prevent or address future pandemics
Benjamin tenOever
In parallel, the tenOever lab tested amodiaquine and hydroxychloroquine against native SARS-CoV-2 in a head-to-head comparison in a small animal Covid-19 model, and saw that prophylactic treatment with amodiaquine resulted in about 70 percent reduction in viral load upon exposure, while hydroxychloroquine was ineffective. They also saw that amodiaquine prevented the transmission of the virus from sick to healthy animals more than 90 percent of the time, and that it was also effective in reducing viral load when administered after introduction of the virus. Thus, their results suggest that amodiaquine could work in both treatment and prevention modes. “Seeing how beautifully amodiaquine inhibited infection in the Airway Chip was extremely exciting,” said Frieman. “And, the fact that it seems to work both before and after exposure to SARS-CoV-2 means that it could potentially be effective in a wide variety of settings.”
“This collaboration has allowed us to do things that we never would have had the resources to do otherwise, including recently setting up Organ Chips in our own lab so that we can now use them to study the interactions between infectious viruses and their hosts. While we’re proud of what we’ve accomplished so far for Covid-19, we’re also looking forward to studying additional virus-host dynamics using the Organ Chips in the hopes that we’ll be able to prevent or address future pandemics,” said tenOever, who is a professor of microbiology.
A preprint of the amodiaquine results was published online, which generated buzz in the scientific community. It eventually caught the eye of Medicines for Malaria Venture, a leading product development partnership in antimalarial drug research. These results, along with studies from several other groups, contributed to amodiaquine’s inclusion in a clinical trial in collaboration with the University of Witwatersrand in South Africa and Shin Poong Pharmaceutical in South Korea last fall. A few months later, the Drugs for Neglected Diseases Initiative (DNDi) added amodiaquine to the ANTICOV clinical trial for Covid-19, which spans 19 sites in over 13 different countries in Africa. Amodiaquine is oral, extremely inexpensive, and widely available in Africa. If proven effective in these clinical trials, it could provide a badly needed weapon against Covid-19 in low-resource nations where access to vaccines and expensive new therapeutics is limited.
While the identification of amodiaquine is a major boon in fighting Covid-19, the team already has their sights set on future pandemics. In addition to SARS-CoV-2, their recent publication details their success in finding drugs that could protect against or treat several strains of influenza virus. “Thanks to our experience using this drug development pipeline to validate amodiaquine for Covid-19, we are now applying what we learned to influenza and other pandemic-causing pathogens,” said co-author Ken Carlson, a lead senior staff scientist who helps lead the Coronavirus Therapeutic Project Team at the Wyss Institute. “This process has given us confidence that Organ Chips are predictive of what we see in more complex living models of viral infections, and helped harness the creative cauldron of the Wyss Institute to consolidate and strengthen our therapeutic discovery engine.”
In addition to influenza, the team is now exploring drugs that could be used against the new SARS-CoV-2 mutant strains, to suppress the dangerous “cytokine storm” that leads to many hospitalizations, and to relieve the symptoms of Covid-19 “long haulers.”
Recommended article
News • Deadly mechanism uncovered
Inside COVID-19's 'cytokine storm'
Leading immunologists in Japan are proposing a possible molecular mechanism that causes massive release of proinflammatory cytokines, or a cytokine storm, leading to the acute respiratory distress syndrome (ARDS) in COVID-19 patients. Their suggestions, published in the journal Immunity, are based on recent findings that explain how SARS-CoV-2 enters human cells.
“The pandemic has really gelled the Wyss Institute’s Bioinspired Therapeutics development program, and linking up with the Frieman and tenOever labs has created a drug discovery and development pipeline that dramatically speeds up the whole process, quickly shepherding Covid-19 drugs through preclinical development to the point where they can be tested in humans. With Organ Chip technology in hand, we are now in a stronger position to confront future pandemics,” said Ingber, who is also the Judah Folkman Professor of Vascular Biology at Harvard Medical School and Boston Children’s Hospital, and Professor of Bioengineering at the Harvard John A. Paulson School of Engineering and Applied Sciences.
Source: Wyss Institute at Harvard University
04.05.2021