Breast cancer
Olwen Glynn Owen reports on MammaPrint, a test that pinpoints the risk of breast cancer recurrence from the primary tumour's genetic make-up. Women with breast cancer tumours under <20mm are usually considered by clinical guidelines to have a good prognosis.
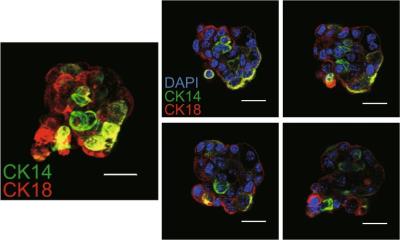
However, results of a small study, reported at the recent European Society for Medical Oncology (ESMO) congress in Stockholm, Sweden, suggest that for a substantial proportion the recurrence risk and later distant metastases is much higher than previously thought.b In their study, researchers at the Netherlands Cancer Institute, Amsterdam, and the Bioinformatics division of Agendia Laboratories, used the latter’s microarray gene-expression analysis tool, MammaPrint, to assess whether tumour tissue showed gene profiles indicating a high or low risk of metastasis. The MammaPrint test compares how many of a reference set of 70 key genes linked to breast cancer and have been shown to influence risk
of recurrence, are present in a tumour’s gene signature.
Lead author Dr Annuska Glas of Agendia, said that, of 319 small tumours analysed with MammaPrint, 39 were below 11mm and of these 19 (49%) showed a good prognostic gene signature with a predicted 10-year overall survival of >90%. The remaining 20 (51%) had a poor prognostic gene signature and their 10-year distant metastasis-free survival (DMFS) was calculated as being only 60%. In the 280 patients with tumours between 11 and 20mm, the probability of remaining free of distant metastases at 10 years was 85% in the group with a good prognostic signature (44%) and 60% in the group with a poor prognostic signature (56% of patients). In a further sample of tumours from 145 patients of indeterminate clinical risk (lymph node-negative, oestrogen receptor-positive and grade 2), 90 (62%) had a good prognostic signature with a predicted 10 year survival of 92% while 55 (38%) had a poor prognostic signature with a 10-year projected survival of 60%.
Even when tumours in the study were below 11mm, patients at high risk could be identified by their tumour gene signature in comparison to the reference gene set in MammaPrint, said Dr Glas. Results of the test have important implications for how women are then treated over the next 10 years and choice of any adjuvant therapy selected for them. The test can also predict tumour sensitivity to specific chemotherapy agents. Patients identified by the test as high risk can be pre-tested to see what treatments offer their particular cancer the best chance of tumour response, progression free survival and a good outcome. The MammaPrint DNA microarray will complement other tests, such as TargetPrint used to decide which patients are likely to respond best to certain targeted therapies MammaPrint results provide extra guidance for oncologists over clinical guidelines on whether or not adjuvant therapy is required, said Dr Glas. The test has been independently validated in several cohort studies and is claimed to be a more reliable predictor than tests involving standard prognostic markers.
Identifying patients at low risk is as important as identifying those at high risk for purposes of deciding on adjuvant chemotherapy. Oncologist Michael Wolf of Vivantes Klinikum am Urban, Berlin, Germany, said: ‘Compared with the St Gallen criteria, MammaPrint identifies significantly more patients (approx 40% vs 15%) with a lower recurrence risk.’
Molecular testing with sophisticated assay technology is an essential component in new moves to offer patients treatment tailored specifically to their individual cancer rather than treatments that have been demonstrated to benefit average patients in large groups, said pathologist Professor Giuseppe Viale, pathologist at Milan University, and a director of the European Institute of Oncology. Basing decisions on results of tests like MammaPrint was introducing a whole new philosophy, he said: ‘It’s a new paradigm where we are listening to what individual tumour cells tell us they need to transform, grow, progress and survive and then choosing treatments targeting those processes.’
Prof Rene Bernards, Agendia CEO, claims the test is superior to conventional means of assessing risk of future metastatic disease such as histological assessment of tumour aggressiveness, and has been well validated. The fresh tumour samples used in Mamma Print testing are considered more effective at yielding RNA-based information than paraffin-fixed samples in conventional laboratory testing yielding degraded RNA.
MammaPrint’s FDA submission was supported by a study in women up to age 60. Tumour samples and clinical data from 302 patients under 61 at five European centres in the TRANSBIG study confirmed the test was useful in predicting time to distant metastasis in the two earliest stages of the disease (Stage I and Stage II) where tumour size is equal to or less than 50 mm and when the cancer is lymph node negative. A prospective study MINDACT (Micro-array In Node-negative Disease may Avoid Chemotherapy) is now comparing the relative accuracy of MammaPrint against traditional prognostic assessment methods in predicting the outcomes of 6,000 patients, focusing on patients where there is discordant assessment between the two methods.
‘Around 70% of all breast cancer patients with stage 1 or II disease could stay free of metastases without any adjuvant therapy. ‘It’s important to identify this group accurately,’ said Dr Joseph Gligorov, oncologist at Tenon Hospital, Paris, who estimated that cutting out unnecessary treatment would improve quality of life and save ?50,000 per patient.
• Approved by European regulators in 2005, MammaPrint became the first FDA-approved in vitro diagnostic multivariate index assay (IVDMIA) device and was cleared for USA use in 2007. It is now used in centres worldwide.
19.11.2008