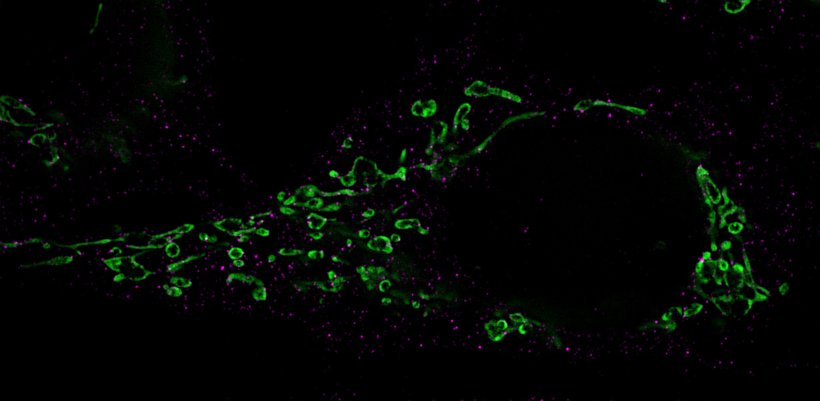
Image source: Lehrstuhl für Virologie / Universität Würzburg
News • HHV awaken via microRNA
Why herpes keeps coming back
Eight different herpes viruses are known to date in humans. They all settle down permanently in the body after acute infection. Under certain circumstances, they wake up from this dormant phase, multiply and attack other cells.
This reactivation is often associated with symptoms, such as itchy cold sores or shingles. In the course of evolution, most herpesviruses have learned to use small RNA molecules, so-called microRNAs, to reprogram their host cells to their advantage. A research team led by Bhupesh Prusty and Lars Dölken from Julius-Maximilians-Universität (JMU) Würzburg in Bavaria, Germany, has now been able to show for the first time that a viral microRNA acts as a master regulator to induce the reactivation of the virus. In the journal Nature, the researchers present the previously unknown cellular mechanism by which human herpesvirus 6 (HHV-6) triggers its own awakening.
More than 90 percent of all people are infected with HHV-6 without noticing it. The virus probably only causes problems when it wakes up repeatedly. HHV-6 reactivation is suspected of impairing heart function, causing the rejection of transplanted organs and triggering diseases such as multiple sclerosis or chronic fatigue syndrome (ME/CFS). In addition, recent studies suggest that this herpesvirus may be involved in the development of schizophrenia, bipolar disorder and other diseases of the nervous system. "How herpesviruses reactivate from a dormant state is the central question in herpesvirus research," says JMU virologist Lars Dölken. "If we understand this, we know how to intervene therapeutically." A previously unknown key to this is a viral microRNA called miR-aU14. It is the central switch that initiates the reactivation of HHV-6.
Recommended article

News • Sexually transmitted infections
New findings could improve STI vaccinations
In a new study, researchers from King’s College London have shown how skin vaccination can generate protective CD8 T-cells that are recruited to the genital tissues and could be used as a vaccination strategy for sexually transmitted infections (STIs), such as HIV or herpes simplex virus.
The regulatory miR-aU14 comes from the virus itself. As soon as it is expressed, it interferes with the metabolism of human microRNAs. In doing so, it selectively interferes with the maturation of several microRNAs of the miR-30 family. As a result, these important cellular microRNAs are no longer produced. This in turn affects a cellular signaling pathway, the so-called miR-30 / p53 / Drp1 axis. Through this pathway, the viral miR-aU14 induces mitochondrial fragmentation. These cell structures are of central importance for energy production, but also for signal transmissions in the defense against viruses.
The viral miR-aU14 thus interferes with the production of type I interferons – messenger substances with which the cell signals the presence of viruses to the immune system. Because the interferons are missing, the herpesvirus is able to switch from a dormant to an active state undisturbed. Interestingly, the Würzburg research group was also able to show that the viral microRNA is not only essential for virus replication, but also directly triggers the reactivation of the virus from its dormant state.
The researchers now want to understand the exact mechanism by which the viral microRNA initiates the reactivation of the virus. In addition, there are first indications that other herpesviruses can also be reactivated via the same mechanism. This could reveal therapeutic options to either prevent reactivation of these viruses or to specifically trigger it in order to then eliminate the reactivating cells. Another goal is to understand the molecular consequences of mitochondrial fragmentation in detail.
For the first time, this work from Würzburg shows that a microRNA can directly regulate the maturation process of other microRNAs. This also opens up new therapeutic possibilities: Artificial small RNAs can be designed to specifically switch off individual members of microRNA families. Such subtle interventions were not possible until now.
Source: University of Würzburg
07.05.2022