Image: C. Gatsogiannis / MPI für molekulare Physiologie
News • ClpX-ClpP protein complex
Weak spot in pathogens could be key in new antibiotics
Antibiotics are still the most important weapon for combatting bacterial infections. But medical science is running out of “ammunition” because of more and more frequently occurring resistances.
A research team has now elucidated the structure of the proteolytic complex ClpX-ClpP. This is a key to development of innovative antibiotics which target the degradation process of defective proteins in bacteria. Almost 700,000 people in Europe suffer from infections every year through antibiotic-resistant pathogens; approximately 33,000 of them die. Despite this enormous and globally increasing danger, very few new antibiotics have been developed and approved in the past few decades. There is no improvement in sight. That is why it is urgently necessary to find new points of attack in pathogenic bacteria and to develop new antibiotics which exploit these weak spots.
Recommended article

Article • Differentiate and select
Myths and truths about antibiotics, antiseptics and vaccination
Sixty-two percent of Germans fear antibiotic resistance, according to a survey recently conducted by the German Federal Institute for Risk Assessment. ‘Patients colonised by multi-resistant pathogens are particularly scared. But many of these fears are rooted in misunderstandings,’ explained Professor Mathias Pletz at the Congress for Infectious Diseases and Tropical Medicine (KIT).
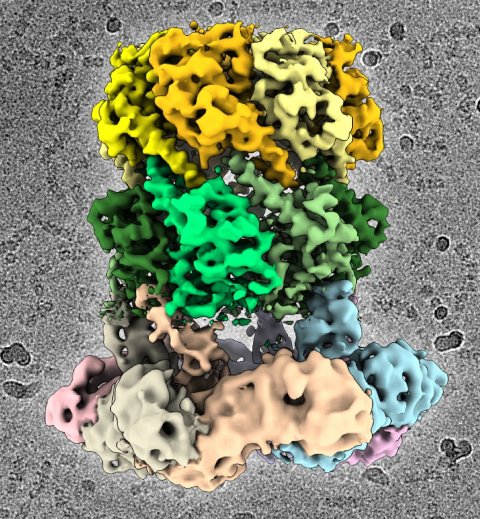
Image: C. Gatsogiannis / MPI für molekulare Physiologie
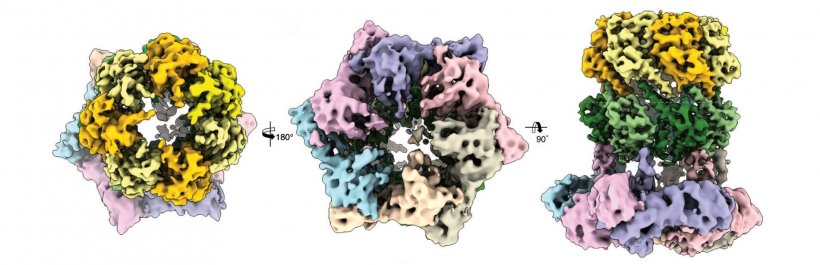
A particularly promising point of attack for antibacterial therapies is the proteolytic enzyme ClpP: on the one hand it plays an important role in bacterial metabolism, and on the other hand it ensures the controlled degradation of defective proteins. But for this purpose it requires the ClpX protein as a starting aid. In the complex with ClpP, ClpX identifies proteins which should be degraded, unfurls them and guides them into its barrel-like degradation chamber.
Scientists in the groups led by Prof. Stephan Sieber, Technical University of Munich (TUM) and Prof. Stefan Raunser, Director at the Max Planck Institute of Molecular Physiology in Dortmund, have now elucidated the three-dimensional structure of the ClpX-ClpP proteolytic complex for the first time and thereby established an important basis for future pharmacological applications.
A new class of potential antibiotics – the so-called acyldepsipeptide (ADEP) antibiotics – also brings about an uncontrolled degradation through ClpP without the support of ClpX. As a result also vital proteins are destroyed – with lethal consequences for the bacteria. This unique mechanism of action has considerable innovation potential in the fight against pathogenic bacteria. Whereas common antibiotics act through the inhibition of vital processes, in this case the antibacterial effect is achieved through the activation of a process.
Recommended article

Article • Medication development
Support from the other end of the world
Partners who could hardly be further apart – yet have a lot in common – have united to fight resistant pathogens. The International Consortium for Anti-Infective Research (iCAIR) is based in Germany and Australia – separated by nearly 16,000 km as the crow flies. This has not stopped the research cooperation from achieving its objectives: the development of new agents against infections.
In addition to the degradation of defective proteins, ClpP is also a decisive regulator in the production of an arsenal of bacterial toxins which are primarily responsible for the pathogenic effect of many pathogens. At the TUM, the group led by Prof. Stephan Sieber has been successfully researching the ClpP protease for years, and has already developed a large number of potent inhibitors against ClpP and ClpX which stop the production of bacterial toxins and can therefore more or less disarm them. Dóra Balogh has now managed to produce and stabilize the ClpX-ClpP complex.
But up to recently the structure of the ClpX-ClpP complex could not yet be elucidated in detail. Dr. Christos Gatsogiannis, researcher in the group led by Prof. Stefan Raunser at the MPI of Molecular Physiology, has now managed this by means of cryogenic electron microscopy. With this technology they were able to demonstrate that ADEP and ClpX dock onto ClpP at the same spot, but control the process of protein degradation in a different way: Whereas ClpX does not lead to an alteration in the structure of ClpP, ADEP brings about an unintended opening of the complex. As a result, intact proteins are also degraded in an uncontrolled manner and without the support of ClpX.
The clarification of this mechanism by the research teams from Dortmund and Munich is a milestone on the way to the development of innovative antibiotic substances targeting ClpP.
Source: Technische Universität München (TUM)
07.10.2019