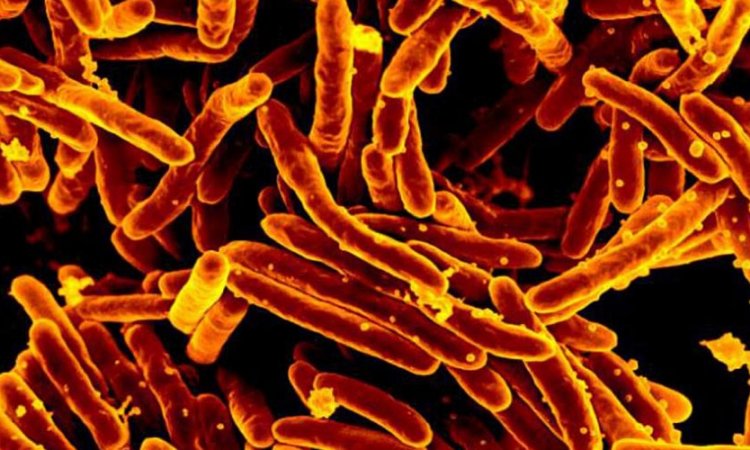
News • TB drug development cooperation
Unite4TB: A new era in Tuberculosis treatment development
To advance anti-tuberculosis (TB) science and enable the progression of new, safe, and affordable treatment solutions for TB patients worldwide, a new consortium of 30 partners from 13 countries has officially launched.
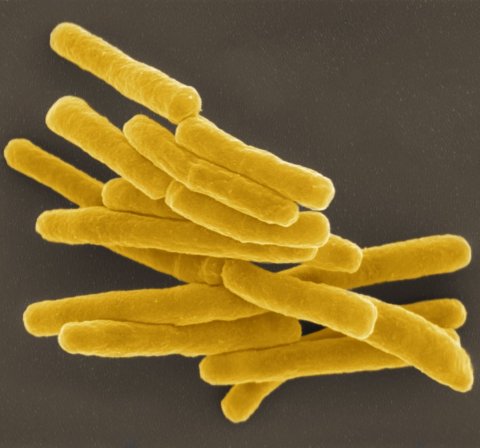
Image source: RKI/Gudrun Holland; colouration: Andrea Schnartendorff
The 7-year, €185 million project called Unite4TB, aims to accelerate and improve the clinical evaluation of combinations of existing and novel drugs, with the goal of developing new and highly active TB treatment regimens for drug-resistant and -sensitive TB. Unite4TB is the newest project of the IMI AMR Accelerator, a public-private collaboration with the shared goal of progressing the development of new medicines to treat or prevent resistant bacterial infections. “Tuberculosis is a major threat to public health worldwide. By bringing together leading experts from the public and private sectors in Europe and beyond, Unite4TB is well placed to deliver results that will accelerate the development of better treatment regimens to tackle this disease,” says Dr Pierre Meulien, Executive Director of IMI.
Worldwide, TB is one of the top 10 causes of death and the leading cause from a single infectious agent (above HIV/AIDS). The growing emergence of multidrug-resistant TB is well-recognised as a public health challenge and has sparked new interest and investment in anti-TB drug development. Despite increased activity in the field, an integrated approach to TB drug development is still limited.
Recommended article
News • Bioorganic chemistry
Why M. tuberculosis is so resistant to drugs and immune defenses
A consortium of researchers from Russia, Belarus, Japan, Germany and France led by the Skolkovo Institute of Science and Technology have uncovered the way in which Mycobacterium tuberculosis survives in iron-deficient conditions by utilizing rubredoxin B, a protein from a rubredoxin family that play an important role in adaptation to changing environmental conditions.
With European Federation of Pharmaceutical Industries and Associations (EFPIA) and Associated Partners on board, Unite4TB has access to the majority of the most innovative TB compounds, currently in late pre-clinical, clinical phase 1, and early phase 2 stage. The consortium will deliver an efficient, global clinical trials network equipped to conduct phase 2 trials. State-of-the-art adaptive trial designs will be implemented, and advanced modelling, artificial intelligence and machine learning techniques will be employed. All of this will allow for the selection and testing of novel combination regimens with a high probability of success in subsequent phase 3 clinical trials.
I am confident that Unite4TB will contribute towards achieving the goal of ending tuberculosis by 2030
Anja Karliczek
Anja Karliczek, Germany’s Federal Minister of Education and Research, says: “Europe’s Unite4TB project creates an important new platform for research to combat tuberculosis. Science and industry will jointly test their clinical candidates and share research results. The objective is to develop effective combinations for new, urgently needed solutions to treat tuberculosis. This public-private partnership will set a new standard in the fight against global diseases such as TB. Unite4TB is a remarkable example of international research collaboration. I am delighted that Germany is supporting the consortium with funding of around 25 million euros to the two German Associated Partners. I am confident that Unite4TB will contribute towards achieving the goal of ending tuberculosis by 2030 that was adopted by the G20 Heads of State and Government at the UN General Assembly.”
Unite4TB is the largest public-private collaboration on clinical TB drug development in the history of the EU. It will set a new standard for anti-TB regimen development, enhancing the efficiency with which new treatments are delivered to TB patients across the world.
Source: Unite4TB
15.07.2021