News • Novel immune checkpoint discovered
Targeting the cancer microenvironment
Researchers discover a novel checkpoint in immune cells with the potential to treat the cancer cell microenvironment
The recognition of bacterial infections or foreign substances is mediated and controlled by the human immune system. This innate and adaptive immune system comprises the most important metabolic and cellulare processes to fight against infections and other diseases. Paradoxically, this immune system is also involved in the development of systemic diseases and cancer. Therefore it is of utmost importance to further understand fundamental biochemical processes involved in the cellular reactions of the immune system which can lead directly to novel therapies against systemic diseases and cancer.
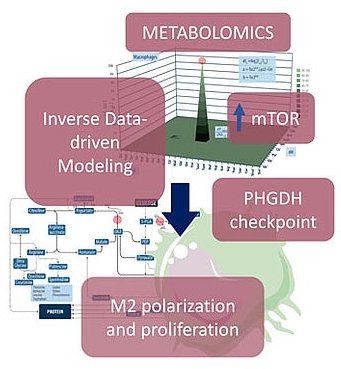
Image source: University of Vienna
The enormous complexity of the immune system comprises different cell types. Two types, M1- and M2-type macrophages are involved in inflammatory defense mechanisms (M1) but also healing processes (M2). These M2-type macrophages are also known to associate with cancer cells and supporting their growth (Tumor-associated macrophages, TAM´s). The mechanism by which these cell types can differentiate into each other is called the polarization process.
In the new study, the research teams of the system biologist and biochemist Wolfram Weckwerth and the immunologist Thomas Weichhart have found a novel biochemical switch involved in the polarization and proliferation of M2 macrophages. This biochemical checkpoint branches from the glycolytic pathway – the major pathway in human metabolism for glucose breakdown – to provide molecular units for rapid cell proliferation and differentiation. If this pathway is inhibited by specific substances the M2 polarization and proliferation is downregulated. This has direct consequences for the understanding of TAMs and the treatment of cancer. „Our hypothesis is that if we inhibit the growth of M2-type macrophages in the microenvironment of cancer cells we could revoke the supportive effect for cancer growth. This biochemical checkpoint which we found in these M2-type macrophages is exactly the point for targeting their growth inhibition“, says Wolfram Weckwerth.
The two labs of Thomas Weichhart and Wolfram Weckwerth are now intensively working to study this mechanism in more detail and to develop potential strategies for cancer cell treatment. Central for this project is the Vienna Metabolomics Center which enables the thorough study of these complex metabolic processes of the human immune system and cancer metabolism by combining metabolite profiling and data-driven computational modelling. The Vienna Metabolomics Center was also recently involved in a study for cancer treatment by specific combinations of fasting and drug administration leading to reduced tumor growth.
Source: University of Vienna
05.02.2020