Image source: ICMAB-CSIC
News • Bioengineered scaffolds
New hydrogel to improve CAR T immunotherapy
Researchers of the Institute of Materials Science of Barcelona (ICMAB-CSIC) and the Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS)-Hospital Clínic de Barcelona have developed novel lymph-node-inspired hydrogels that significantly enhance the activation, gene expression, and proliferation of CAR T cells.
This could optimize the production of CAR T cells, a promising immunotherapy for cancer, by providing a more efficient and cost-effective platform for cell manufacturing. The study, published in ACS Applied Materials & Interfaces, highlights the potential of bioengineered scaffolds in advancing the field of cell-based therapies.
CAR T cells (Chimeric Antigen Receptor T cells) are immune cells genetically modified to recognize and destroy cancer cells. By equipping T cells with synthetic receptors (CARs) that target specific molecules on tumor cells, scientists can create a living, patient-specific therapy capable of attacking cancer with precision. This personalized form of immunotherapy has already shown success in treating certain types of leukemia and lymphoma and is being actively researched for broader applications.
By recreating key aspects of the lymph node microenvironment, our hydrogels provide biochemical and mechanical cues necessary for enhanced CAR expression and T cell proliferation
Judith Guasch
The ICMAB team, in collaboration with IDIBAPS-Hospital Clínic researchers, has now engineered innovative poly(ethylene glycol)-heparin hydrogels with interconnected pores and tissue-like stiffness, mimicking the architecture of lymph nodes—where T cells naturally activate and proliferate. These hydrogels have demonstrated a significant increase in CAR expression and T cell proliferation compared to traditional suspension cultures. In lab tests, these hydrogels increased the percentage of CAR+ cells by 50% and doubled the replication index compared to conventional suspension cultures, a significant boost in both quality and quantity of therapeutic cells.
“By recreating key aspects of the lymph node microenvironment, our hydrogels provide biochemical and mechanical cues necessary for enhanced CAR expression and T cell proliferation,” explains Judith Guasch, group leader of the Dynamic Biomaterials for Cancer Immunotherapy group at ICMAB and corresponding author of the study. “This approach not only has the potential to improve the current CAR T cell products, but also to reduce manufacturing costs, making therapies more accessible.”
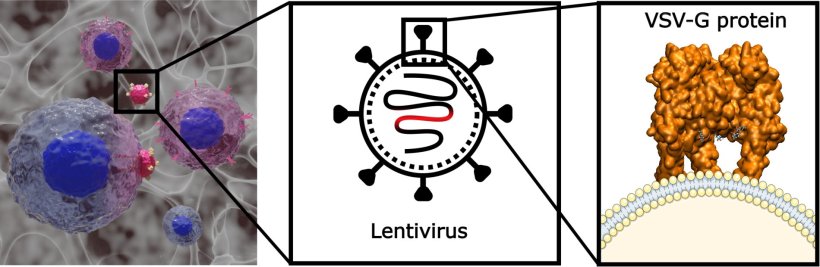
Image source: ICMAB-CSIC
To generate CAR T cells, scientists need to introduce the CAR gene into T cells. This is typically done using lentiviral vectors, a type of engineered virus that safely delivers genetic material into cells. However, this gene transfer process is a complex step with variable yields in CAR T cell therapy.
In this study, theoretical simulations led by Jordi Faraudo, from the Theory & Simulation group at ICMAB and corresponding author of the study, showed that heparin, a negatively charged component of the hydrogel, electrostatically binds to the positively charged VSV-G proteins that cover the viral particles, and increases their interaction with T cells. This enhances gene transfer efficiency, leading to a higher number of T cells successfully expressing the CAR. “Our simulations indicate that heparin interacts with lentiviral particles through strong electrostatic interactions and bring them into closer proximity with T cells, facilitating their interaction with these cells and enhancing CAR gene delivery,” says Faraudo.
This synergy between materials science and theoretical modeling offers a new way to boost CAR T cell production without altering the viral vectors themselves—simply by improving the environment in which gene delivery takes place.
These findings underscore how bioengineered hydrogels can help make CAR T cell therapies more efficient, scalable, and accessible—potentially expanding their clinical use in oncology and beyond. This interdisciplinary collaboration highlights the importance of combining biomaterials engineering with theoretical modeling to advance cell-based therapies.
Source: Institute of Materials Science of Barcelona; Anna May
16.04.2025