Image source: University of Illinois; image by Beckman Imaging Technology Group
News • Strain mapping and antimicrobial properties
Smart coatings give early warning for implant failure
Newly developed “smart” coatings for surgical orthopedic implants can monitor strain on the devices to provide early warning of implant failures while killing infection-causing bacteria, University of Illinois Urbana-Champaign researchers report.
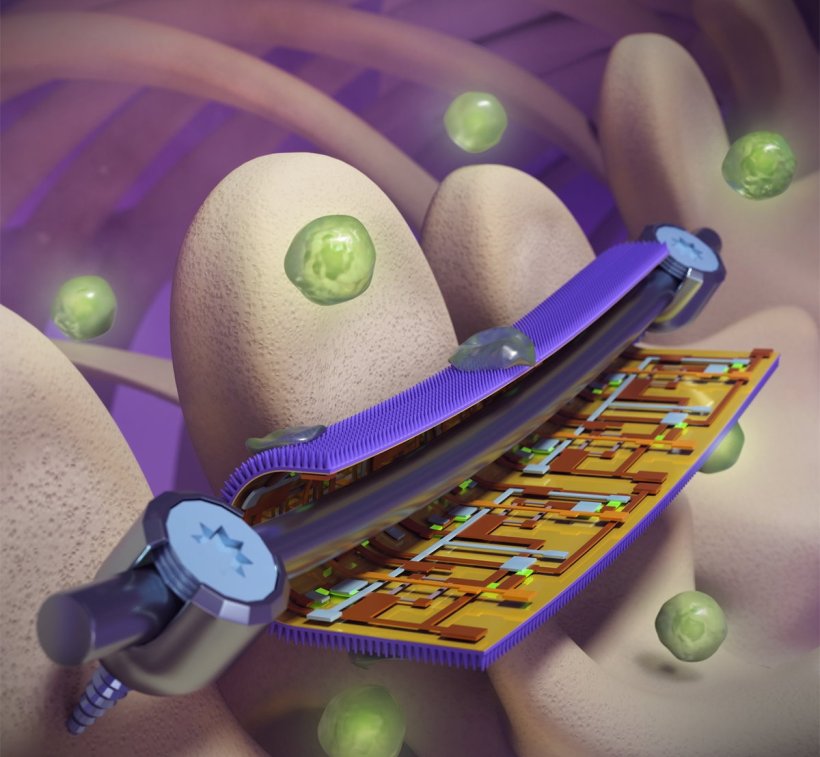
The coatings integrate flexible sensors with a nanostructured antibacterial surface inspired by the wings of dragonflies and cicadas.
In a new study in the journal Science Advances, a multidisciplinary team of researchers found the coatings prevented infection in live mice and mapped strain in commercial implants applied to sheep spines to warn of various implant or healing failures. “This is a combination of bio-inspired nanomaterial design with flexible electronics to battle a complicated, long-term biomedical problem,” said study leader Qing Cao, a U. of I. professor of materials science and engineering.
Using a mechanical approach to killing bacteria allowed us to bypass a lot of the problems with chemical approaches, while still giving us the flexibility needed to apply the coating to implant surfaces
Gee Lau
Both infection and device failure are major problems with orthopedic implants, each affecting up to 10% of patients, Cao said. Several approaches to fighting infection have been attempted, but all have severe limitations, he said: Biofilms can still form on water-repelling surfaces, and coatings laden with antibiotic chemicals or drugs run out in a span of months and have toxic effects on the surrounding tissue with little efficacy against drug-resistant strains of bacterial pathogens.
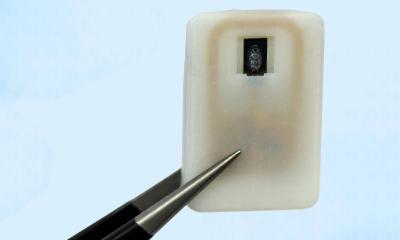
Taking inspiration from the naturally antibacterial wings of cicadas and dragonflies, the Illinois team created a thin foil patterned with nanoscale pillars like those found on the insects’ wings. When a bacterial cell attempts to bind to the foil, the pillars puncture the cell wall, killing it. “Using a mechanical approach to killing bacteria allowed us to bypass a lot of the problems with chemical approaches, while still giving us the flexibility needed to apply the coating to implant surfaces,” said pathobiology professor Gee Lau, a coauthor of the study.

Image source: University of Illinois; photo by Yi Zhang
On the back side of the nanostructured foil, where it contacts the implant device, the researchers integrated arrays of highly sensitive, flexible electronic sensors to monitor strain. This could help physicians watch the healing progress of individual patients, guide their rehabilitation to shorten the recovery time and minimize risks, and repair or replace devices before they hit the point of failure, the researchers said.
The engineering group then teamed up with veterinary clinical medicine professor Annette McCoy to test their prototype devices. They implanted the foils in live mice and monitored them for any sign of infection, even when bacteria were introduced. They also applied the coatings to commercially available spinal implants and monitored strain to the implants in sheep spines under normal load for device failure diagnosis. The coatings performed both functions well.
The prototype electronics required wires, but the researchers next plan to develop wireless power and data communications interfaces for their coatings, a crucial step for clinical application, Cao said. They also are working to develop large-scale production of the nanopillar-textured bacteria-killing foil. “These types of antibacterial coatings have a lot of potential applications, and since ours uses a mechanical mechanism, it has potential for places where chemicals or heavy metal ions – as are used in commercial antimicrobial coatings now – would be detrimental,” Cao said.
Source: University of Illinois
08.05.2023