News • Glioblastoma
Researchers block protein to stop brain tumors' self-repair
Researchers at the San Diego branch of the Ludwig Institute for Cancer Research at University of California San Diego, with colleagues around the country, report that inhibiting activity of a specific protein in glioblastomas (GBM) boosts their sensitivity to radiation, thus improving treatment prospects for one of the most common and aggressive forms of brain cancer.
The findings are published in Cancer Cell.
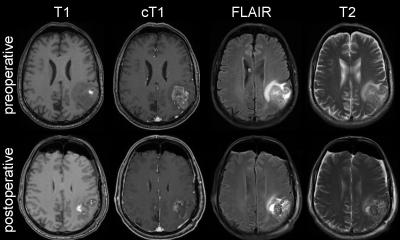
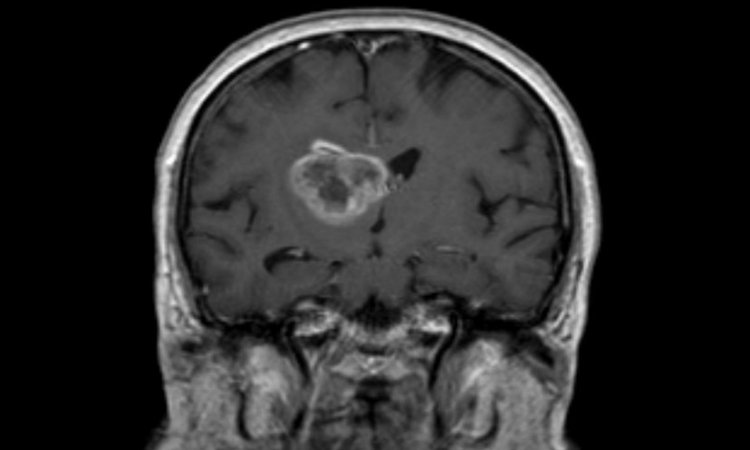
GBM are extremely difficult to treat, with a median survival rate of 15 to 16 months, meaning that half of all patients survive to this length of time. Radiation and chemotherapy are the standard-of-care treatments, both intended to damage and destroy DNA in cancer cells, but their efficiency declines as tumors develop therapeutic resistance.
In their new paper, senior author Frank B. Furnari, PhD, professor of pathology at UC San Diego School of Medicine and member of Ludwig San Diego, first author Jianhui Ma, PhD, a postdoctoral fellow, and colleagues identified a mechanism used by GBM to promote therapeutic resistance: phosphorylation of a protein called phosphatase and tensin homolog or PTEN, which is encoded by the PTEN gene.
These findings are novel and provide a foundation to move forward with a clinical trial and hopefully expand our findings to other cancer types that use this mechanism to evade therapy
Frank B. Furnari
Normally, PTEN acts as a tumor suppressor, but mutations or modifications can produce the opposite effect. Specifically, the researchers found that phosphorylation of PTEN — the adding of a phosphate molecule to an amino acid called tyrosine Y240 in cancer cells — promoted DNA repair in tumors, reversing the effects of therapeutic radiation. When scientists blocked Y240 phosphorylation in mouse models of GBM using inhibitors of the fibroblast growth factor receptor, the cancer cells became sensitive to radiation and more died, extending survival in the mice. “These findings are novel and provide a foundation to move forward with a clinical trial and hopefully expand our findings to other cancer types that use this mechanism to evade therapy,” said Furnari.
The work follows up on a 2017 study, published in Nature Communications, in which Furnari and colleagues elucidated how PTEN regulates development of GBMs.
Source: University of California San Diego
04.03.2019