Molecular imaging in clinical practice
By Lutz S. Freudenberg MD MBA, of ZRN Grevenbroich and the Nuclear Medicine Department at the University of Duisburg-Essen, and Thomas Beyer of cmi-experts GmbH, in Zurich, describe nuclear medicine at work
Over the past decades new imaging technologies have substantially broadened the range of imaging applications in clinical medicine. For years anatomical imaging modalities, such as X-ray and CT, reveal high-resolution information of organs and tissues over extended imaging ranges. Lately, however, the idea of functional imaging e.g. the visualisation of physiology in vivo gains importance.
In the last decade, ‘Molecular’ imaging is the buzzword that implies a new era of imaging. It connotes a multi-disciplinary field of research and applications dedicated to in situ visualisation of biological molecules and molecular biology. As such, the idea of molecular imaging is to unite molecular biology and in vivo imaging. It enables the visualisation of the cellular function and the follow-up of the molecular process in living organisms without perturbing them. This would lend itself to accurate diagnosis of virtually all common human diseases, such as cancer, neurological and cardiovascular diseases. The basic advantage and thrill of the molecular imaging approach is not to visualise the effects of certain diseases on organs and functions but the causes ‘at work’.
To date clinical molecular imaging requires the injection of a tracer, an imaging agent with a signal traceable from the outside. Molecular imaging agents are diverse and numerous, and include most of the radiopharmaceuticals used in routine nuclear medicine as well as experimental tracers used with X-ray, CT, MRI, optical imaging, and ultrasound in clinical and preclinical trials. However, radiopharmaceuticals used for scintigraphy, SPECT or PET are by far the predominant agents of molecular imaging. The founding myth about the tracer method is revealed in the early life Nobel Laureate George von Hevesy who was living in a boarding house as a poor doctoral student in 1911. He suspected his landlady of reusing food left over from his dinner plate. One day Hevesy decided to add a radiotracer (212-Pb) to his dinner leftovers. When he detected (aka traced) radioactivity in his meal the following day, he accused his landlords of recycling his food. He was right. However, as a consequence of his wizardry he was thrown out of the boarding house.
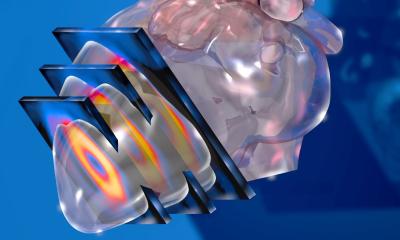
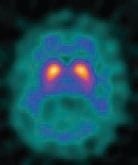
Von Hevesy’s tracer method is the basic concept of nuclear medicine (and molecular imaging) today and has been the cornerstone of clinical nuclear medicine. Over the years, radionuclide detectors have become exceptionally sensitive to small amounts of radioactivity. Collimated SPECT imaging system typically provide sensitivities of 2-4 counts/s/mCi of technetium-99m, while standard PET imaging systems typically have sensitivities of 2,000-5,000 counts/s/mCi of fluorine-18. These high sensitivities can be used to detect picomolar concentrations of tracers and permit mapping of metabolism, perfusion, receptor expression and dynamic imaging of compartments. Within years, functional imaging of the thyroid, skeleton and heart (fig. 1) – the most frequent examinations – are increasing; emphasising the importance in clinical practice and decision making (fig. 2 & 3). Furthermore, these imaging techniques are the framework for radionuclide therapies, such as radio-iodine therapy or radiopeptide therapy. Together with the development of new tracers molecular imaging techniques become more important and help establish the concept of individualised treatment planning by using new dosimetric concepts. In short: Molecular imaging is at work in clinical practice all over the world and many patients have benefited and will benefit in the future.
Today, molecular imaging has entered a new phase by combining nuclear medicine and radiology imaging technologies into a single, integrated concept: dual-modality imaging. Nuclear medicine methods, such as SPECT and PET have the intrinsic ability to detect diseases earlier than, for example, anatomical imaging alone. CT, as the most widely used anatomical tomographic imaging technique, visualises and measures morphology and structure.
Diseases are then detected and depicted through changes of the anatomical tissue distributions. While CT has a very high spatial resolution, it is limited by a very low functional resolution, i.e. active tumour tissue and necrosis frequently cannot be separated on CT alone. Thus, a combination of the CT and PET or SPECT appears synergistic. Through accurate spatial (and temporal) co-registration both the specificity and the sensitivity of SPECT, PET and CT images can be increased considerably. From a recent meta-analysis of the improvements in cancer staging, Czernin et al. derived an overall 10-15% improvement in staging and restaging accuracies of FDG-PET/CT over FDG-PET, or CT alone, for a variety of cancers. In particular, patients with head and neck cancer, thyroid cancer, breast cancer, lung cancer, cancers of the gastrointestinal tract, cancer of unknown primary, lymphoma, and melanoma benefit from integrated FDG-PET/CT (Fig. 3).
The future will show whether the recently proposed combination of PET and MRI will be able to live up to these standards presented by PET/CT in oncology, or if novel, yet untouched applications can be addressed effectively by combined PET/MRI.
The near future of nuclear medicine, and therewith molecular imaging, will benefit from new tracers that help to visualise receptor-status (figure 4) and hypoxia. In addition to the benefits to patients, hybrid imaging can help overcome professional boundaries, such as those between radiology and nuclear medicine, with the objective of an interdisciplinary approach to optimise diagnosis and treatment planning further, to prepare healthcare systems for the next decades.
06.03.2010