Video • Miniscule swimmers
Microrobots could re-shape drug delivery
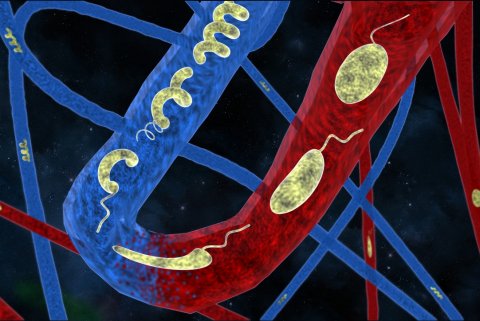
Scientists have developed minute flexible robots that could help revolutionise drug delivery in the future. These ‘microrobots’ are so small that they could be ingested, or inserted into human veins to deliver drug therapies directly to diseased body areas.
Report: Mark Nicholls

The microbot project is still very much at the research stage, but scientists masterminding the research at ETHZ (Swiss Federal Institute of Technology Zürich) and EPFL in Lausanne are confident it has enormous potential in the delivery of specialised treatments. The highly-flexible biocompatible microrobots – or microswimmers – have been developed by scientists led by Professor Brad Nelson at ETHZ and Professor Selman Sakar at EPFL. With tiny magnets within the design, the machines – which vary in size from a few millimetres down to less than a millimetre in total length – can swim through liquids and change shape as needed to move through narrow blood vessels and intricate systems.
© 2019 EPFL/ETHZ
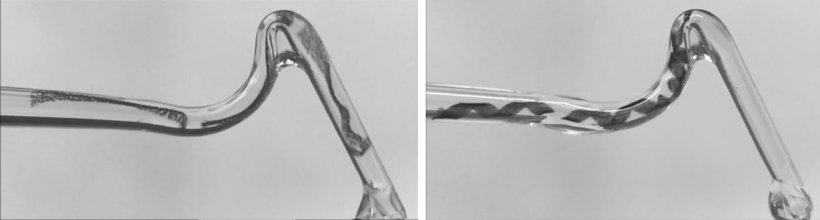
Additionally, the microswimmers can be of different shapes or stiffness to reflect a specific task, with the study team following a variant of origami, called kirigami, to design and fold compliant 3-D microstructures from a thermo-responsive gel composite reinforced with micronanoparticles.
In the robot design, Nelson, who is Professor of Robotics and Intelligent Systems in the Department of Mechanical and Process Engineering at ETH Zürich, said: ‘We were inspired by tiny microorganisms, like bacteria, which can change their shape to escape physical traps. To control and manoeuvre them, we embed magnetic particles in the materials and then externally generate magnetic fields to create forces on them to propel them through liquid.’
© 2019 EPFL/ETHZ
To create the robot devices, he explained that the team used stimuli-responsive hydrogels made of N-Isopropylacrylamide and polyethylene glycol diacrylate with iron-oxide magnetic nanoparticles embedded within them. The magnetic nanoparticles allow them to be controlled via an electromagnetic field, though the microrobots have been designed in such a way that they can also utilise the fluid flow to navigate on their own through cavities.
In their findings the researchers suggest that the ‘development of microscopic artificial swimmers that can cross biological barriers, move through bodily fluids, and access remote pathological sites can revolutionise targeted therapies.’ Professor Nelson added: ‘In nature, there is a multitude of microorganisms that change shape as their environmental conditions change. We have been inspired by this basic principle in the development of our microrobots. The key challenge for us was to develop the physics that describe the types of changes we were interested in, and then to integrate this with new fabrication technologies,’ he pointed out adding that, in terms of healthcare, particularly in respect of targeted drug delivery, ‘They are able to swim through tortuous blood vessels to deliver drugs, for example to unblock a thrombosis or to fill an aneurysm.’
Additionally, Sakar, Assistant Professor in the Institute of Mechanical Engineering at EPFL, said: ‘Our robots have a special composition and structure that allow them to adapt to the characteristics of the fluid they are moving through. For instance, if they encounter a change in viscosity or osmotic concentration, they modify their shape to maintain their speed and manoeuvrability without losing control of the direction of motion.’
While currently at the research stage, the next step for the scientists is to test them in ex vivo animal tissue. However, they remain confident that their robots can be inexpensive and be manufactured relatively easily.
Profile:
Brad Nelson has been Professor of Robotics and Intelligent Systems at ETH Zürich since 2002, conducting research on microrobotics, nanorobotics, and medical robotics. His key interest lies in how to make tiny intelligent machines of millimetres to nanometres size, with applications in medicine. Nelson was an Assistant Professor at the University of Illinois at Chicago and an Associate Professor at the University of Minnesota before moving to ETH. With over 30 years of experience in the field of robotics, he has received numerous awards for his work in robotics, nanotechnology, and biomedicine.
18.07.2019