Image source: Adobe Stock/SciePro
News • Oncology & Immunology
Study shows mechanism of PTEN-deficient breast cancer
A group of researchers led by the Dana-Farber Cancer Institute, Boston, has illustrated the mechanistic pathway for PTEN-deficient breast cancer and successfully tested a method of countering the downstream effects of immune system evasion by tumors.
The study, "PI3Kβ controls immune evasion in PTEN-deficient breast tumours," published in Nature, details the relationship between the general tumor suppression of PTEN and the specific actions of enzyme PI3Kβ when PTEN is inactive. Anghesom Ghebremedhin and Judith A. Varner have published a News and Views article discussing this work in the same journal.
Higher risk of breast cancer with PTEN mutations
Women with PTEN mutations have a 400% higher risk of breast cancer than those without, and PTEN-related cancers are more resistant to treatment. The researchers looked at the downstream factors of PTEN inactivity on cancer growth and immunotherapy evasion, which led them to a key suspect, the enzyme PI3Kβ.
PI3Kβ enzymes are involved in the signaling pathways that affect cell growth, proliferation, migration, secretion, differentiation, transcription and translation - all things a healthy cell needs to do. However, like any enzyme in the body, it is only needed when it is needed, and the functional levels of PI3Kβ enzyme activity are regulated by PTEN activity. When the PTEN gene is missing or mutated in a way that limits its activity, the proliferation of PI3Kβ activity can result in tumor formation.
PTEN gene mutation effects
The researchers made this discovery in a mouse model with PTEN-deficient breast cancer. With PTEN missing, PI3Kβ promoted tumor growth by setting off a cellular survival and proliferation strategy in the cancer cells and activated an immunosuppression response with a transcription factor called STAT3.
Following the mechanism chain of events backward, researchers found that PI3Kβ inactivation in a PTEN-null mouse model reduced STAT3 signaling and increased the expression of immune stimulatory molecules, thereby promoting the anti-tumor immune responses. Pharmacological PI3Kβ inhibition also elicited this anti-tumor immunity response and synergized with immunotherapy to inhibit tumor growth.
Mice that successfully responded to the combined treatment by becoming cancer-free later displayed an immune system memory response that effectively rejected tumor reintroduction.
The research demonstrates a molecular mechanism linking PTEN loss and STAT3 activation in cancer and linking PI3Kβ to immune evasion in PTEN-null tumors. The study authors suggest this provides a rationale for combining PI3Kβ inhibitors with current immunotherapy in treating PTEN-deficient breast cancer.
The results will likely have a significant impact as PTEN gene mutation effects reach far beyond breast cancer and are heavily linked to uterus, skin, prostate, thyroid, and kidney cancers. The current study is identifying causal mechanisms as opposed to just correlations of events, meaning the findings could quickly be followed up by clinical applications.
Single-cell RNA-Seq analysis
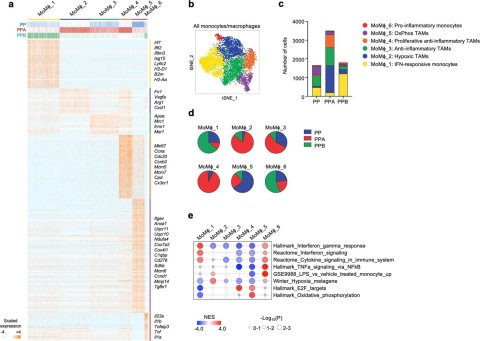
Single-cell RNA-Seq analysis of monocytes/macrophages cluster in PTEN/p53-null breast tumors with catalytic isoform-specific PI3K deletion. Single-cell RNA-Seq analysis of monocytes/macrophages (MoMϕ) cluster from PP, PPA and PPB tumors (n = 3 mice per group).
a Heatmap showing gene expression for top 10% differentially-expressed genes (DEGs) for each MoMϕ sub-cluster. Selected genes are indicated on the right. Genotype of the tumor of origin is indicated on top.
b tSNE of all MoMϕ analyzed denoting sub-cluster.
c MoMϕ sub-cluster representation per genotype.
d Genotype representation per MoMϕ sub-cluster.
e GSEA of MoMϕ sub-clusters. Statistical significance of enrichment scores (p-values) were calculated by GSEA using a phenotype-based permutation test.
Source: Science X Network
28.04.2023