Source: Pixabay/TBIT
News • Histones & protamines
Infertility's roots may lie in our DNA
Pathological infertility is a condition affecting roughly 7% of human males, and among those afflicted, 10-15% are thought to have a genetic cause. However, pinpointing the precise genes responsible for the condition has been difficult, due to the extensive number involved in generating and developing sperm cells.
In a new paper appearing in Science Signaling, a Japanese team reports unravelling the mechanism behind one cause of infertility - incomplete development of the proteins packaging DNA in sperm cells - and further, success in making test mice fertile by replacing a single amino acid on a key protein. In every cell, thread-like DNA is wound tightly in the nucleus around bobbin-like proteins called 'histones'. And in sperm cells - the smallest humans produce - another protein called 'protamine' is needed to wind the strands even tighter. "It's been known for about 30 years that protamines are modified and matured during sperm development to enable proper functioning," explains first author Katsuhiko Itoh from Kyoto University's Graduate School of Medicine. "This prompted us to disclose the underlying mechanism and biological consequences of protamine regulation, so that we can see how this process contributes to 'spermatogenesis' - the making sperm cells."
Source: Kyoto University/Itoh Lab
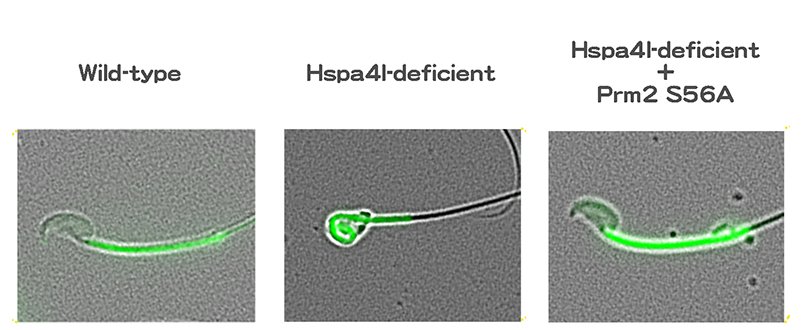
The team focused on a series of chaperones: proteins that assist in processing other proteins. Detailed genetic analysis revealed that a chaperone known as Hspa4l is key to proper sperm cell development, and that its dysfunction has consequences similar to deficiency in a gene called Ppp1cc2. "Further study showed that Hspa4l is vital for the proper functioning of phosphatase Ppp1cc2, a protein regulator," continues Itoh. "A dysfunction in Hspa41 stops Ppp1cc2 from reaching chromatin, resulting in failure to dephosphrylate protamine 2 at serine 56, and with protamine 2 not functioning, sperm cells don't develop."
With these data, the team produced a mouse in which serine 56 was substituted to alanine, dephosphomimetic of dephosphorylated serine: the single amino acid replacement that made all the difference. When combined with a mutation resulting in non-functioning Hspa41, the mouse showed no dysfunction in spermatogenesis, effectively preserving fertility.
Itoh emphasizes that his team's research shows the value in studying protamine modification in the process of sperm cell maturation. The group hopes to further unravel the complex network of gene interaction and protein modification in spermatogenesis, and its further implications for infertility.
Source: Kyoto University
02.04.2019





