News • Infiltrating tumors
Immune cells predict therapy response in breast cancer
When immune cells invade the tumor, this is usually considered a good sign because the body's own immune system appears to be responding to the cancer.
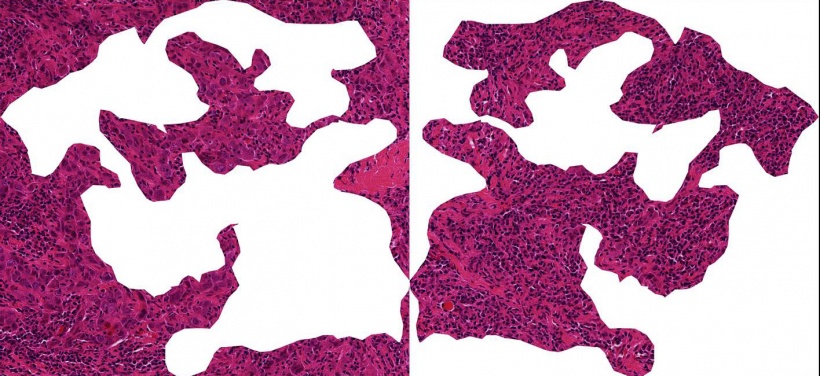
In the case of certain types of breast cancer immune cells, namely so-called tumor-infiltrating lymphocytes (TILs), these can determine survival rates and predict the usefulness of chemotherapy. This was shown by the largest meta study on TIL content to date, conducted by the scientists of the German Cancer Consortium (DKTK) at the Charité - Universitätsmedizin Berlin in collaboration with the German Breast Group. In the German Cancer Consortium (DKTK), the German Cancer Research Center (DKFZ) in Heidelberg joins up with certified German university hospitals specialized in oncology.
The option of chemotherapy can be a difficult one to make when it comes to breast cancer. The treatment can help to reduce tumor size if given before surgery so that less tissue needs to be removed. But it also comes with serious side effects, so that doctors are required to weigh the costs and benefits for each patient. A new study describes tumor-infiltrating lymphocytes (TILs) as an important factor in predicting recovery chances and the usefulness of chemotherapy. Increased TILs indicate that the chemotherapy will be more effective against the tumor.
Chemotherapy and immune system support each other in order to combat the tumor
Carsten Denkert
"Particularly in rapidly growing breast tumors, the presence of immune cells within the tumor tissue is a good indicator of improved response rates. The tumor is then visible to the immune system," explains Carsten Denkert, of the Institute of Pathology at the Charité, who is one of the breast cancer experts in the DKTK. Denkert and his colleagues would like to know how reliably the TIL cell content can be used as a biomarker for various types of breast cancer, as well as which immune cell types are the decisive indicators. In this most comprehensive study to date on immune cell infiltrates in breast cancer the research team showed that there can be major differences between tumor subtypes. The scientists analyzed the amount of tumor infiltrating immune cells in 3771 patient tissue samples from six clinical breast cancer studies. All women had received chemotherapy prior to surgery. The study showed that a high TIL content correlates with an improved response to therapy and improved survival rates in patients with so-called triple negative and HER2-positive breast cancer. "In this situation, the chemotherapy and immune system support each other in order to combat the tumor," said Denkert.
This is different in the case of the luminal breast cancer subtype (hormone receptor-positive/HER2 negative). Patients with this form of breast cancer typically had lower TIL values and in these tumors TILs did not correlate with higher survival rates. "We believe that there is a different type of immunological infiltrate and a different relation with therapy outcome in this type of breast cancer," summarizes Denkert. A closer look revealed that macrophages, which belong to the white blood cells, are indicators of poor prognosis in luminal breast cancer. On the other hand, a high number of antibody-forming B cells, correlates with good survival rates.
"Our results show that the TIL content in certain aggressive forms of breast cancer has a high value in predicting which patients might benefit from chemotherapy," emphasized Denkert. For a further improvement of response rates, new immunotherapies are currently being tested which could additionally activate the immune system against the tumor. The DKTK partner site Berlin, in collaboration with the German Breast Group, are conducting biomarker tests in the clinical study GeparNuevo, identify immune cells as as markers for successful immunotherapies with so-called immune checkpoint inhibitors. In the future, TILs could be used as markers to better direct breast cancer treatments for patients.
Source: The German Cancer Research Center (Deutsches Krebsforschungszentrum, DKFZ)
12.12.2017