Image source: MAKY.OREL, Tibia - detail of bone tissue (proximal end) 2, CC0 1.0
News • Orthopaedics
Iliac crest reconstruction: 15 patients recruited into GreenBRIC study
GreenBone Ortho, a company specialising in bone regeneration, announces that it has achieved its aim of recruiting 15 patients into the GreenBRIC study.
GreenBRIC study is a prospective, open label, single-arm, First-in-Human clinical investigation, in male and female patients, aged between 18 and 70 years, undergoing surgery to correct bone defects using GreenBone Implant, specifically for iliac crest reconstruction. The primary objective of this study is to evaluate the safety of GreenBone Implant and the secondary objective is to evaluate its performance. The surgical procedure will be performed as per standard of care at the investigational site. The GreenBRIC study is conducted at the Leeds University, UK and the Principal Investigator is Professor Peter Giannoudis, Professor at the School of Medicine, University of Leeds and head of Trauma & Orthopaedic Department at Leeds General Infirmary (LGI). The study was approved by the Leeds West Research Ethics Committee, HRA (Health Research Authority), MHRA (Medicines and Healthcare products Regulatory Authority).
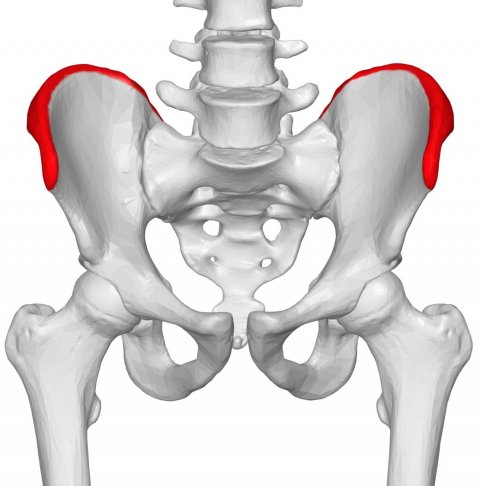
Image source: BodyParts3D is made by DBCLS, Iliac crest 03 - anterior view, CC BY-SA 1.0
Defects of iliac crest most frequently result from harvesting of bone graft. Iliac crest bone grafts are considered the gold standard method in bone grafting procedures and they are widely used in a variety of orthopaedic, neurosurgical, and maxillofacial procedures to promote bone healing and to restore bone defects. Harvested tricortical graft blocks are used to facilitate fusion of joints (i.e. pubis symphysis, sacroiliac joint), to structurally support metaphyseal areas of articular impaction injuries (i.e. tibial plateau) and to fill in metaphyseal bone voids. Noteworthy, defects resulting from harvesting of iliac bone grafts can be associated with significant morbidity and complications including infections, hematomas, hernias, persistent pain and fracture of the ilium.
The GreenBone bone substitute, with its inherent physical and biomimic properties, posseses similar architecture structure to human bone, thus, being a suitable material to be used for the treatment of the bone defect (3- 4cm) induced from the harvested tricortical iliac crest, restoring therefore the pelvic anatomy and minimising the risk of chronic pain, haematoma formation and herniation of the abdominal contents. The preliminary results from this study confirms the safety profile of the GreenBone 3D implant. Prof. P. Giannoudis said: “The GreenBone scaffold was found to be user friendly and easy to apply during surgery. The bone defect in the iliac crest was well covered and continuity of the anatomy was achievable. The scaffold proved to be a safe option to consider for restoration of the bone defect taking into consideration that the other options to use include the allograft and xenograft (bovine) options. There was no increased pain observed but rather based on the patient’s feedback the symptoms claimed were well controlled and by four weeks there was no need for additional pain relief. Quality of life had significantly improved following surgery. The healing process based on the radiological findings were associated with features indicative of scaffold integration and bone restoration. There were features of uniform healing with no signs of breakdown or fragmentation. No device deficiencies have been observed. We are continuing with the followup visits of the enrolled patients”
“We are very pleased to have achieved this important milestone," says CEO Lorenzo Pradella. “And we are confident that the final clinical results will support the market access of the product. We expect the results from the study to offer a safe alternative in the standard of care for bone loss given that our innovative 3D bone graft is proven to be as good as autograft, which requires a second surgical procedure to harvest bone from the patient’s iliac crest. These clinical data will play a key role in our commercial strategy to increase our share of bone graft substitute market in Europe.”
Source: GreenBone
28.07.2021