GE Healthcare at Euroanaesthesia
Clinicians all over the world use GE Healthcare products and solutions to anesthetize patients. The breakthrough ideas of a small Ohio company in 1910, the predecessor to the anesthesia division of GE Healthcare, have evolved into innovations that continue to open new frontiers in anesthesia. Today, GE Healthcare provides anesthesia technologies in many countries worldwide, collaborating closely with clinicians to positively impact the lives of their patients. Siv Schalin, General Manager at GE Healthcare for Finland, said, “We are very pleased to be a part of Euroanaesthesia again this year and privileged to present to clinicians, industry experts and institutions in Helsinki the latest GE Healthcare innovations and highlight our commitment to ‘healthymagination’.”
Innovations on display at Euroanaesthesia
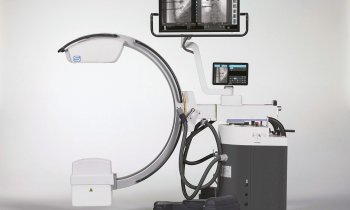
Aisys Carestation with Et Control1
At Euroanaesthesia 2010, GE Healthcare launches in Europe Et Control, to help provide consistent and accurate agent delivery across various patient types. Et Control helps to provide predictable, consistent and accurate delivery of agent and oxygen during anesthesia. With Et Control, digital settings are monitored and adjusted through simplified menus. Et Control is currently available in some markets as an upgrade on the Aisys Carestation. Aisys is built on GE Healthcare’s culture of innovations in anesthesia delivery and patient monitoring technologies.
CARESCAPE Monitor B650
GE Healthcare introduces the CARESCAPE™ Monitor B650, a flexible patient monitoring solution that supports efficient clinical decision-making. The CARESCAPE Monitor B650 will improve data integration and connectivity by directly linking to the GE CARESCAPE portfolio of monitoring products including the latest bedside, central station and transport monitors—as well as existing GE monitors and peripheral third-party devices.
Traditionally, hospitals have implemented patient monitors on a care area by care area basis, making it difficult to connect disparate systems across the clinical setting. To address this challenge, GE Healthcare strives to offer clinicians a solution that gives them access to the right information, when and where they want it. In markets where the B650 will be available, this will consist of an integrated, enterprise-wide patient monitoring platform based around the new, flexible CARESCAPE Monitor B650 and the recently introduced CARESCAPE Monitor B850 for the highest-acuity patients.
The CARESCAPE Monitor B650 combines the strong clinical heritage of Datex-Ohmeda’s anesthesia expertise and Marquette Electronics’ cardiac expertise. To preserve patient monitoring investments already made by GE Healthcare customers, the CARESCAPE Monitor B650 will be compatible with many existing Marquette and Datex-Ohmeda products.
Anesthesia Delivery Management
Whether one practices inhalational anesthesia, intravenous anesthesia, or local/regional anesthesia, it is important to optimize the delivery of the volatile, hypnotic/opiate and infiltration drugs to the patient. GE Healthcare’s new product innovations are specifically designed to assist the caregiver with this vital task:
For the European market, GE Healthcare’s Anesthesia Delivery Management solutions include:
• Aisys with End-tidal Control (EtC) for target control of volatile agent and patient oxygen.
• Navigator Applications Suite for predictive drug modeling including synergistic interaction with total drug effect display for volatile agents and intravenous drugs
• Surgical Plethysmographic Index (SPI)3 for real time monitoring of hemodynamic responses caused by surgical stimuli and analgesic medications under general anesthesia
• Adequacy of Anesthesia (AOA) for an integrated monitoring view of anesthesia parameters to assist clinicians in the management of sedation and analgesia according to individual needs
• Venue 40 for ultrasound-assisted local and regional anesthesia
• Advanced Breathing System for exceptional breathing circuit kinetics
Anesthesia Workflow Management
In Perioperative care, where high quality information has to be available at the tip of a finger, clinicians can rely on GE Healthcare's new product innovations to provide timely access to clinical information and help enable dependable decision support and more efficient patient status assessment . These solutions are designed to acquire, store and retrieve critical data efficiently and securely, spanning the entire anesthesia care process with data continuity and integrity, allowing the clinician to focus on patient care. Seamless integration with hospital information systems, optimized touch-screen operation and unique flexibility, make them compelling solutions to help manage anesthesia workflow.
For the European market, GE Healthcare’s Anesthesia Workflow Management solutions include:
• CARESCAPE Monitor B850 and CARESCAPE Monitor B650 (coming soon), part
• of the CARESCAPE patient monitoring clinical intelligence solution for Perioperative care
• Centricity Anesthesia - electronic anesthesia assessment , recording and follow-up
• Opera scheduling and theatre management system to optimize the surgical process
Vital Signs Devices
In addition, on display at GE’s booth are Vital Signs Devices. The acquisition of Vital Signs by GE
Healthcare marks a distinct expansion of GE’s vision, capabilities and services. Vital Signs enhances the entire GE Anesthesiology and Respiratory portfolio. Vital Signs Devices complete the connection between equipment and the patient across the wide range of surgical procedures and equipment platforms. “We are celebrating 100 years of innovation - from one of the first commercially available anesthesia machine to the first anesthesia system with target control of volatile anesthetics, where the user sets target levels for end-tidal oxygen and anesthesia agent and the anesthesia system economically achieves and maintains those values. GE Healthcare is committed to providing exceptional and innovative solutions that enable quality, consistent , and efficient patient-focused care,” added Schalin.
1Not commercially available in all markets, not cleared or approved by the U.S. FDA.
2Not CE marked, not cleared or approved by the U.S. FDA, not available in Europe or any other market.
3Not commercially available in all markets, not cleared or approved by the U.S. FDA.
15.06.2010