Image source: NIAID, Novel Coronavirus SARS-CoV-2 (49679608341), CC BY 2.0
News • Coronavirus disease diagnostics
Emergency Use Authorization for COVID-19 test
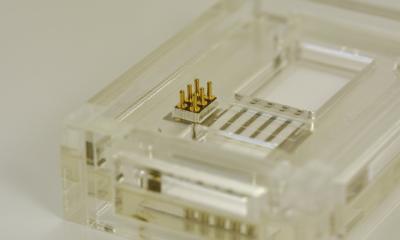
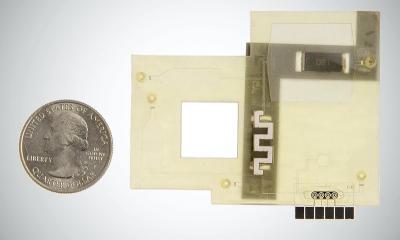
French in vitro diagnostics company bioMérieux announced that its subsidiary, BioFire Defense, has received Emergency Use Authorization (EUA) by the U.S. Food and Drug Administration of its BioFire COVID-19 test for use in CLIA moderate and high complexity clinical laboratories to detect the novel coronavirus SARS-CoV-2.
The test detects SARS-CoV-2 in approximately 45 minutes from a nasopharyngeal swab in transport media. This test runs on the fully automated Filmarray 2.0 and Filmarray Torch platforms and is extremely easy to use, therefore requiring minimal training and skills in molecular biology. BioFire COVID-19 was developed with funding from the U.S. Department of Defense (DoD) by leveraging an existing contract agreement with BioFire Defense. This is the second of three tests being developed for diagnostic use as part of bioMérieux’s strategic response to the COVID-19 pandemic.
Recommended article
News • RT-PCR for COVID-19
First of 3 diagnostic tests for SARS-CoV-2 coronavirus available
Biotechnology company bioMérieux, a world leader in the field of in vitro diagnostics, is announcing the forthcoming launch of 3 different tests to address the COVID-19 epidemic and to meet the different needs of physicians and health authorities in the fight against this emerging infectious disease. bioMérieux has finalized the development of the SARS-CoV-2 R-Gene test. This real-time PCR test…
We are making every effort to provide a comprehensive diagnostic approach that meets the highest performance and quality standards to help physicians mount an effective response to the ongoing COVID-19 pandemic
Mark Miller
“The rapid development of this test is a combined result of the extensive effort and dedication of our employees, the assistance of our partner Midwest Research Institute Global, and the confidence entrusted to us by the U.S. Department of Defense,” said Bob Lollini, CEO of BioFire Defense.
bioMérieux is currently making every effort to scale up supply of the BioFire COVID-19 test at multiple production facilities in Salt Lake City (Utah, USA). The initial test kits are committed to the DoD for redistribution. Test kits will be available for commercial distribution in the United States under the EUA as well as internationally where regulatory approval allows. bioMérieux expects to have maximum production capability within a few weeks to address the needs of the thousands of labs and healthcare professionals using one of the nearly 11,000 BioFire systems worldwide. “In the face of this unprecedented global health crisis, bioMérieux is now launching a second diagnostic test for the detection of SARS-CoV2. True to our commitment to public health we are making every effort to provide a comprehensive diagnostic approach that meets the highest performance and quality standards to help physicians mount an effective response to the ongoing COVID-19 pandemic,” said Dr. Mark Miller, Executive Vice President and Chief Medical Officer of bioMérieux.
bioMérieux has also received authorization to sell the BioFire COVID-19 test External Control Kit. This positive control material may be used for quality control and laboratory verification of the test.
Source: bioMérieux
24.03.2020