DMIST demystified
Cynthia E Keen discusses the controversy evoked by an ambitious 36-month digital mammography trial that has cost the US $26,000,000
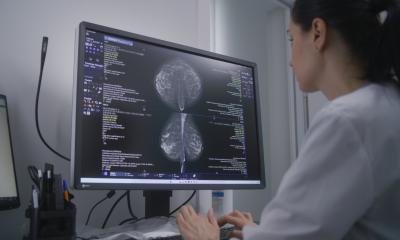
The United States’ Digital Mammographic Imaging Screening Trial (DMIST), like Norway’s Oslo II Study, represents a historic milestone in the adoption of digital mammography for routine use. Yet DMIST has been very controversial. It was criticised and praised from the day that it was funded in 2001, and it was criticised and praised when the first results were published in the New England Journal of Medicine on 16/09/05.
DMIST proved that a digital mammography acquisition system produced superior diagnostic images for radiologist identification of breast cancer in women who have dense breasts, or who are under the age of 50, or who are premenopausal or perimenopausal. Dr Etta D Pisano, DMIST’s principal investigator, said that digital mammography was 14-27 percent more sensitive than film for these three categorical subsets. The images produced of women who do not have these characteristics were equivalent for identifying breast cancer, whether digital or traditional analogue screen mammography systems were used.
So why is DMIST controversial? The study was an aggressive, ambitious, expensive, and bold clinical trial. 49,528 women and 33 medical institutions participated in a 36-month study that cost $26,000,000. Its goal was to determine the value of first generation of digital radiography (DR) and computed radiography (CR) systems commercially available in the world. This included systems that had not yet received government approval for use in the United States. Distinguished US mammographers persuaded the National Cancer Institute to fund the trial of this new-to-market technology to determine if its high cost could be better justified by its superiority in detecting early cases of breast cancer.
Individuals who have breast cancer identified in its earliest stages (phases 0-IIB) have a 75-100% five-year survival rate and significantly lower treatment costs. In 2006, an estimated 1,200,000 people throughout the world will be diagnosed with breast cancer.
The controversy of DMIST is that, in spite of the excellence of its design, the product evolution of both the image acquisition technology and the image processing algorithms advance rapidly. The digital mammography systems that were used became outdated even before the image collection phase of the trial was completed. Thus the results, whether favourable or unfavourable for digital mammography, would be based on the capabilities of a first generation product.
When the first of what will be 15 reports were published in 2005, commercial systems had evolved to their third generation of product development, two systems that were used in the DMIST trial were no longer being sold and, most ironically, the Fuji CR system used for 10,000 mammograms still had not received US Food and Drug Administration (FDA) approval.
Both vendors and current users of the available digital mammography systems believe that digital images produce a superior image due to the ability of improving depiction of low-contrast objects in contrast-detail studies and a wider dynamic range. Digital mammography systems use less radiation, increase equipment utilisation with faster throughput and reduce the number of patient recalls.
The negative is that the DR mammography systems, currently the only type approved for use in the US, are 1.5 to 4 times more expensive than screen film mammography systems. The operational and maintenance costs are also very expensive. In the United States, reimbursement rates for mammograms by both government and insurance companies might not even cover the cost of the exam. To date, only about 8% of US healthcare facilities use digital systems.
The DMIST results have created a dilemma because the installed systems can only image 33% of the women who would benefit from digital mammography. Physicians are starting to recommend that women with a family history of breast cancer have a baseline mammogram at a much younger age, thus increasing the size of the subset. GE Healthcare, Hologic and Siemens Medical Systems are all seeing a steady increase in sales each month since October 2005, although company representatives state that they cannot directly attribute this to the DMIST results. As more women become knowledgeable about the DMIST results, competitive market pressures and fear of malpractice lawsuits may force US healthcare institutions into acquiring digital systems whether they can afford them or not.
When CR mammography is approved in the US, sales are expected to skyrocket. It’s already happening in the rest of the world. Fuji Medical Systems reported that its shipments of CR mammography equipment between April and September 2005 increased 136% from the same term in 2004 and estimates a 160% increase for October 2005-March 2006.
01.03.2006