Credit: Korea Institute of Science and Technology (KIST)
News • Prostate cancer
Diagnosing cancer using a urine test with AI
Prostate cancer is one of the most common cancers among men. Patients are determined to have prostate cancer primarily based on PSA, a cancer factor in blood. However, as diagnostic accuracy is as low as 30%, a considerable number of patients undergo additional invasive biopsy and thus suffer from resultant side effects, such as bleeding and pain.
The Korea Institute of Science and Technology (KIST) announced that the collaborative research team led by Dr. Kwan Hyi Lee from the Biomaterials Research Center and Professor In Gab Jeong from Asan Medical Center developed a technique for diagnosing prostate cancer from urine within only 20 minutes with almost 100% accuracy. The research team developed this technique by introducing a smart AI analysis method to an electrical-signal-based ultrasensitive biosensor.
As a noninvasive method, a diagnostic test using urine is convenient for patients and does not need invasive biopsy, thereby diagnosing cancer without side effects. However, as the concentration of cancer factors is low in urine, urine-based biosensors are only used for classifying risk groups rather than for precise diagnosis thus far.
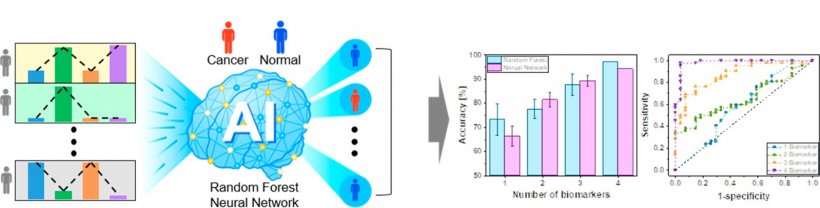
Dr. Lee's team at the KIST has been working toward developing a technique for diagnosing disease from urine with an electrical-signal-based ultrasensitive biosensor. An approach using a single cancer factor associated with a cancer diagnosis was limited in increasing the diagnostic accuracy to over 90%. However, to overcome this limitation, the team simultaneously used different kinds of cancer factors instead of using only one to enhance the diagnostic accuracy innovatively.
The team developed an ultrasensitive semiconductor sensor system capable of simultaneously measuring trace amounts of four selected cancer factors in urine for diagnosing prostate cancer. They trained AI by using the correlation between the four cancer factors, which were obtained from the developed sensor. The trained AI algorithm was then used to identify those with prostate cancer by analyzing complex patterns of the detected signals. The diagnosis of prostate cancer by utilizing the AI analysis successfully detected 76 urinary samples with almost 100 percent accuracy.
"For patients who need surgery and/or treatments, cancer will be diagnosed with high accuracy by using urine to minimize unnecessary biopsy and treatments, which can dramatically reduce medical costs and medical staff's fatigue," Professor Jeong at Asan Medical Center said. "This research developed a smart biosensor that can rapidly diagnose prostate cancer with almost 100 percent accuracy only through a urine test, and it can be further used in the precise diagnoses of other cancers via a urine test," Dr. Lee at the KIST said.
Source: National Research Council of Science & Technology
26.01.2021