Colon Cancer-Detecting Blood Test Previewed at Medica
The future of colon cancer screening may be surprisingly simple. ColoMarker™, an innovative blood test to detect the early stages of colon cancer is generating plenty of attention from a wide audience, including from some of the world’s leading researchers in cancer biomarkers and gastrointestinal medicine.
EDP Biotech, a US-based medical research company, is introducing ColoMarker to medical professionals from around the globe who are attending Germany’s famous Medica trade fair. Dr. Herbert Fritsche, recently retired Chief of the Clinical Chemistry Section of M.D. Anderson Cancer Center, is one of the world’s leading experts on cancer biomarkers as well as a member of EDP Biotech’s scientific advisory board. Dr. Fritsche is attending the trade fair to discuss development of the serum-based test for the early detection of colon cancer.
Another internationally-famous physician, Dr. Gene Overholt, credited as the inventor of the colonoscopy, has also recently joined the company’s scientific advisory board.
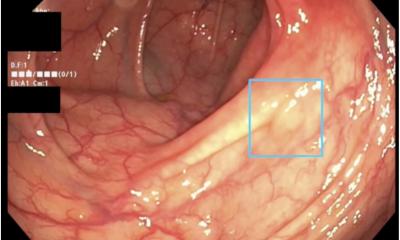
To date, physicians have relied on colonoscopies and fecal occult blood testing (FOBT) to identify potential cases of early stage colon cancer. However, the unpleasantness and discomfort associated with colonoscopies and the limited reliability of other screening tests cause many patients to avoid testing.
“When patients avoid testing, they also avoid the opportunity to detect the cancer at its earliest and most survivable stages,” says Tom Boyd, CEO of EDP Biotech. “This means patients are truly putting their lives at risk. We saw an opportunity to save lives if we could help physicians better identify which of their patients were most in need of a colonoscopy or other testing.”
ColoMarker provides that function as a simple blood test. The test identifies elevated levels of a biomarker in the bloodstream associated with the presence of colorectal cancers. When an elevated result is received, physicians can consult with their patients on the need for additional testing to aid in a diagnosis, prognosis, or treatment plan.
Pre-clinical testing of ColoMarker has shown a greater than 99% accuracy rate for detecting colon cancer in the Stages I, II, and III.
The company also was recently awarded a grant from the United States government for work on further development and testing of ColoMarker. The Qualifying Therapeutic Discovery Project (QTDP) grant is awarded to assist bio-tech companies showing significant potential in their work to develop new therapies and making advances in the fight to cure cancer. By debuting ColoMarker in the exhibit halls of the Medica trade fair, EDP Biotech hopes to bring that same awareness of ColoMarker’s potential to research laboratories and medical practices across Europe.
23.11.2010