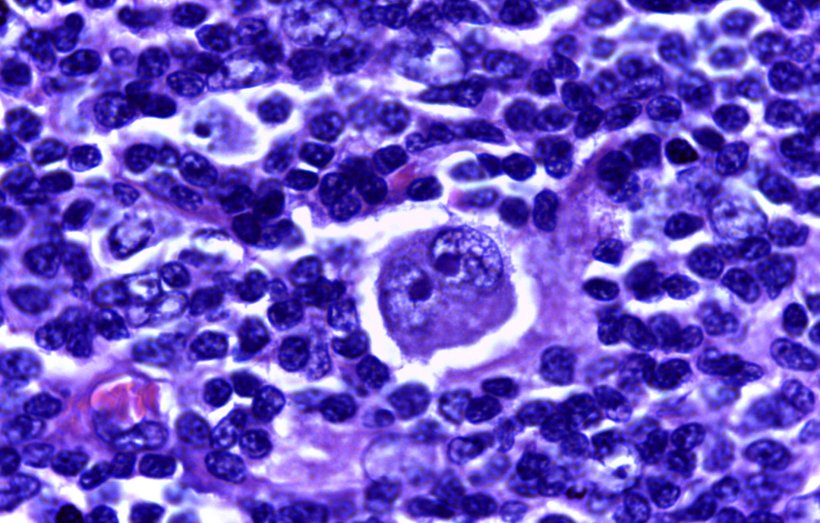
Image source: Calicut Medical College institution QS:P195,Q5019886, Hodgkin's lymphoma 40X, (CC BY-SA 4.0)
News • Reduction of severe side-effects
A 'kinder' chemotherapy for Hodgkin lymphoma
A simple change to the chemotherapy regimen for people with Hodgkin lymphoma could reduce the long-term health impacts that can result from treatment, according to researchers in Cambridge.
The findings could lead to the national guidance on chemotherapy treatment for these patients being revised. The study, published in The Lancet Oncology, was led by Cambridge University Hospitals NHS Foundation Trust (CUH) and the Wellcome Sanger Institute. It compares the lasting effects of two chemotherapy regimens used to treat Hodgkin lymphoma in younger adults. Hodgkin lymphoma is often diagnosed in younger people (age 20-40) so kinder treatments have the potential to deliver significant benefits, such as reduced hospital time and greater likelihood of recovering fertility.
Data previously collected from 1,945 patients treated with the existing chemotherapy regime (eBEACOPP) was compared to 312 patients treated with a similar regimen, called eBEACOPDac. Both treatments use combinations of drugs, and the change replaces one of these, procarbazine, with another called dacarbazine. Both chemotherapies achieved the same success in treating cancer (93.3% in remission 3 years after treatment), but comparison of data from the two groups showed that patients treated with eBEACOPDac generally experienced fewer, less severe side effects.
Recommended article
Article • Focus on treatment and research news
Chemotherapy: effective against cancer, at a cost
Chemotherapy is used against various cancers and is often consideres as a last resort – especially if the cancer has metastasised. Since chemotherapy agents can cause severe side effects, research aims to make treatment more tolerable. Keep up-to-date with the latest research news, medical applications, and background information.
A similar drug substitution is already approved for use in children and is increasingly widely used for the treatment of adults, but this is the first study to rigorously examine its impact in adult patients.
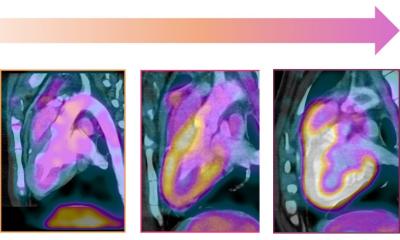
Patients treated with the new regimen spent less time in hospital, required fewer blood transfusions following treatment, and more patients showed signs of recovering fertility sooner. This also has the potential to reduce hospital admissions and demand for hospital appointments. Part of the study used whole genome sequencing at the Wellcome Sanger Institute to look at the effects of both treatments and showed that eBEACOPDac has a greatly reduced impact on patient genes.
Hodgkin lymphoma is a rare, treatable blood cancer. Around 2,000 people per year are diagnosed in the UK and treatment success is high, with over 95% of younger patients cured with treatment. The occurrence of Hodgkin lymphoma in younger people means there is a significant need to reduce the long-term health and fertility impacts of treatment.
Chemotherapy is a well-established approach for treating various cancers, including Hodgkin lymphoma. There are many different chemotherapies consisting of several different drugs used in combination. Chemotherapies are highly effective for treating cancer but also have well-known side effects (e.g. nausea and hair loss) and can have lasting effects following treatment, including anaemia and infertility.

Image credit: Cambridge University Hospitals NHS Foundation Trust
A commonly used standard first-line treatment for people with advanced Hodgkin lymphoma involves chemotherapy known as eBEACOPP. The new eBEACOPDac regimen does not increase the cost of treatment and is administered to patients in the same way. Making eBEACOPDac the recommended treatment for Hodgkin lymphoma in adults could improve the long-term health of patients and enable more of them to go on to have children.
Professor George Follows, Consultant Haematologist at Addenbrooke’s Hospital and the Department of Haematology, University of Cambridge and co-lead author on the study, said: “Our findings highlight the potential to make the short and long-term side effects of chemotherapy much kinder for Hodgkin lymphoma patients without compromising the effectiveness of treatment. By making a small change to how patients are managed, we can greatly reduce the lasting impacts that this disease, and its treatment, has on their lives giving many more patients the opportunity to go on to raise families.”

Image credit: Onur Pinar / Wellcome Sanger Institute
Dr Raheleh Rahbari, Wellcome Sanger Institute and co-lead author on the study, said: “This is an example of how genomics can impact lives and help change healthcare. Through the use of genome sequencing we’ve gained a deeper insight into the lasting effects of chemotherapies, allowing us to learn more about their role in long-term health, and make progress towards effective treatments that minimise side effects as much as possible.”
Dr Cathy Burton, Chair of the UK Hodgkin lymphoma study group, and Haematology Consultant at Leeds Teaching Hospitals NHS Trust, said: “This excellent work provides strong evidence of the benefits of using eBEACOPDac for treatment of Hodgkin lymphoma. This approach of switching procarbazine to dacarbazine is preferable due to its reduced side effects and improvements in fertility recovery. Crucially, these findings are of international significance and should be used to inform treatment guidelines globally to ensure patients are receiving the best treatments.”
This work is illustrative of the benefits that can be delivered through the effective translation of research into clinical practice, which will be further strengthened through the new Cambridge Cancer Research Hospital. The next steps for this research will include more long-term follow up of patients treated with eBEACOPDac, and Professor Follows hopes it will inform a global change in the guidance for treating adults with Hodgkin lymphoma.
The study was co-led by Professor George Follows, Consultant Haematologist at CUH and Dr Raheleh Rahbari, Cancer Research UK Career Development Fellow at the Wellcome Sanger Institute. The clinical research was co-ordinated by Dr Anna Santarsieri, Haematologist at CUH, supported by the Anglia Ruskin University MD Programme. The research was also supported by the Addenbrooke’s Charitable Trust (ACT), Wellcome and National Institute for Health Research Cambridge Biomedical Research Centre (NIHR Cambridge BRC).
Source: Cambridge University Hospitals NHS Foundation Trust
12.12.2024