© TERM / UKW
News • Opportunity for new osteoarthritis treatments
Cartilage regeneration: From the nose to the knee
Treating knee joint defects with cartilage from the nose: The University Hospital in Würzburg is working on the approval of this procedure. They received funding of 2.3 million euros for this purpose.
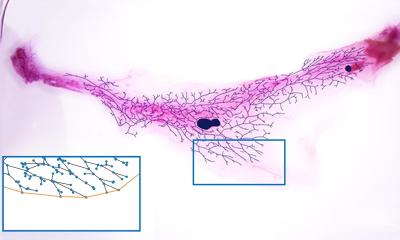
Even though the name might imply it: the recently launched EU project ENCANTO ("magic" in Spanish) does not deal in magic tricks. "We take a small piece of cartilage from the nasal septum of our patients, culture it on a structurally supportive collagen matrix, and implant it four weeks later into the damaged knee to regenerate the cartilage," Dr. Oliver Pullig explains.
The safety and efficacy of this method for cartilage regeneration has already been demonstrated by the biologist in a former nasal cartilage study with an international team under the direction of the University Hospital Basel on more than 100 individuals. In this study, focal cartilage lesions, i.e., locally limited, and clearly defined injuries, which occur for example, after an accident, were treated with the laboratory grown cartilage tissue from the nose. Patients with more advanced cartilage defects will now be included in the ENCANTO study for the first time. It will be investigated whether the procedure represents an alternative to prosthesis and thus a new therapy for patellofemoral osteoarthritis (PFOA), i.e., cartilage damage on the back of the kneecap and the opposing thigh bone. Hence the acronym ENCANTO: ENgineered CArtilage from Nose for the Treatment of Osteoarthritis – artificially produced cartilage from the nose for the treatment of degenerative joint wear.

© TERM / UKW
For the implementation of the ENCANTO project, a total of 11.3 million euros is made available as part of the EU funded program HORIZON-HLTH-2023-TOOL-05 (Tools and technologies for a healthy society). For this endeavor the University Hospital Würzburg receives 1.88 million euros. Spearheading the production of the implants is the dedicated team of the "GMP-compliant ATMP development" led by Oliver Pullig, in collaboration with counterparts from University Hospital Basel. The innovative cartilage matrix will be used across 12 clinical centers in Europe, including the Orthopedic Department of the University Hospital Würzburg.
Another project for the treatment of PFOA is funded by the Swiss National Science Foundation with 2.6 million Swiss francs, of which the University Hospital receives 415,000 euros for production according to Good Manufacturing Practice (GMP). "With these substantial funding amounts, which finally allow us to prepare the product for approval, we have reached the Champions League," says Oliver Pullig, eagerly anticipating the start of recruitment towards the end of 2024 for the Swiss-funded study and at the onset of 2025 for ENCANTO. In Würzburg, the plan includes a total production of 56 implants and the recruitment of 25 patients.

© Kirstin Linkamp / UKW
Although the implantation itself is relatively simple, the effort required to culture the cartilage outside the human body is immense. Because the implant consists of living cells, it is categorized as Advanced Therapy Medicinal Product (ATMP), which means it’s subject to special regulations. "We have already tackled the complex tasks and formalities related to production and logistics in the BIO-CHIP project,” reports Oliver Pullig. Now the challenge lies in consistently maintaining the high standards for production and product quality. "These are human cells. They don't always behave as we desire or expect," Dr. Sarah Nietzer explains. With a decade of work experience in research and development at the department, Nietzer has now transitioned to production and regulation. "We need more data to understand why, for example, the cells from one person don't grow as well as those from another. Additionally, we are working on a method to monitor the quality and viability of the cells throughout the entire production process, in real-time, and not just at the end. It would be fantastic if we could also apply this new method to other models used to simulate various diseases in the department.
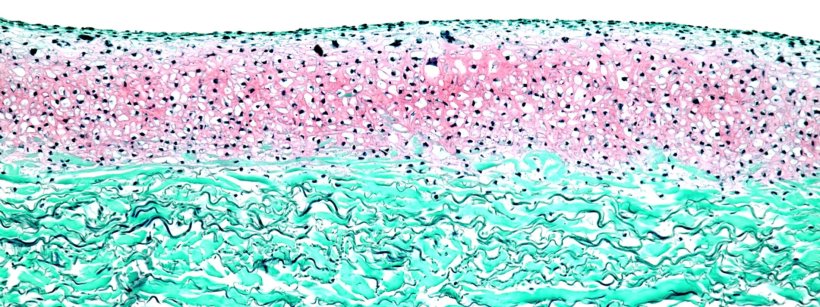
But how is such a tissue implant actually created? Initially, a tiny piece of cartilage tissue is taken from the nasal septum of the study participants. Nasal cartilage cells are very similar to those of the knee; they exhibit mechanical resilience and can be efficiently multiplied in the laboratory. Following the removal a small piece of nasal septum, the cartilage tissue is processed under the strictest aseptic conditions in the cleanroom. The cells are isolated and cultured, and eventually placed onto a 4 x 5 centimeter carrier structure. Here, they migrate into the collagen membrane approved as a medical device, and grow their own cartilage matrix. After four weeks, the implant named N-TEC (nasal chondrocyte-based tissue-engineered cartilage) is ready for use.
"In the nasal study, we also investigated a less time-consuming procedure, where the cells were cultured on the matrix for two days only. The quality was satisfactory, but the more matured matrix was more stable. It was also preferred by the surgeon, who in the end, places the new tissue made from autologous cells onto the defective area in the cartilage and sutures it with the intact cartilage tissue," reports Oliver Pullig. Notably, no autologous blood is needed for cell cultivation anymore, and two matrices can be produced instead of one. This advancement also allows for the treatment of large areas of cartilage defects.
According to Pullig, if the implants prove to be a viable alternative to prosthetics, they would revolutionize the treatment of cartilage degeneration. Thus far, therapeutic approaches have been limited to pain management or artificial joint replacement. Globally, over 500 million individuals suffer from the painful and debilitating effects of arthritis in the knee joint. As obesity rates continue to climb and life expectancy on the rise, so too does the prevalence of this common disease.
Source: Universitätsklinikum Würzburg
25.02.2024