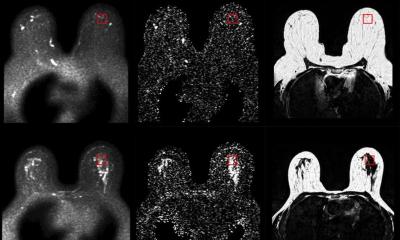
Image source: SBI
Article • Alternative to lumpectomy
Breast cryoablation for surgically inoperative patients
Breast cryoablation is an emerging treatment for early-stage, localized breast cancer that destroys malignant tumours by freezing them. During the past decade, it has been increasingly utilized as an alternative to lumpectomy, but its long-term benefits compared to other breast cancer treatments are still unproven.
Report: Cynthia E. Keen
Breast cryoablation is a non-invasive, less than 60-minute-long outpatient procedure performed with a patient awake under local anaesthesia. The treatment causes fewer complications than breast-conservation surgery, has minimal impact on surrounding breast tissue, has less post-op pain management, and much better cosmesis. It is much less expensive, because it eliminates the need for operating room staff and equipment and requires less post-operative clinical follow-up. In addition to treating early-stage breast cancer treatments, it is used to destroy benign breast lesions and fibroadenomas, to treat patients with Stage IV metastatic breast cancer, and to treat patients unsuitable for surgery.
Case review

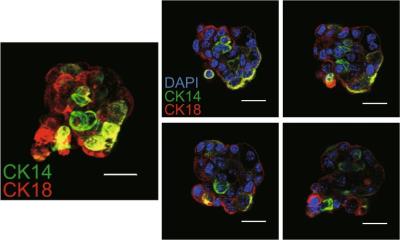
Image source: University of Michigan
Sarah E.H. Moorman, MD, a breast imaging fellow at the University of Michigan School of Medicine in Ann Arbor, presented the hospital’s experiences with breast cancer patients for whom surgery would present a high risk at the recent Society of Breast Imaging annual meeting in Savannah, Georgia. In addition to discussing patient demographics and outcomes, she explained the procedure and offered tips to make the treatment easier for both radiologists and patients. Moorman reported that the findings of the study also showed that this was a safe and effective procedure for women who do not meet the criteria of the largest multi-institutional breast cryoablation clinical trial (ICE3) currently being conducted.
The 16 patients in the study ranged in age from 57 to 94. All had comorbidities that made them poor surgical candidates, including prior stroke and serious cardiovascular conditions, advanced liver disease, COPD, emphysema, interstitial lung disease, and/or high risk of deep vein thrombosis and pulmonary embolism. To identify candidates for breast cryoablation, a surgical oncologist used surgical risk calculators while conducting preoperative evaluations, and then discussed the cases with a radiologist. In addition to surgical risks, the physicians discussed the odds of patients surviving longer with proactive management of their breast cancer, and those for whom anti-endocrine therapy may not be effective.
None of the patients met the all-inclusion criteria used by the ICE3 trial, an ongoing clinical trial evaluating the safety and efficacy of liquid nitrogen-based cryoablation in 194 patients aged 60+ years. The ICE3 participants had unifocal, ultrasound visible low-to-intermediate stage invasive ductal carcinoma 1.5 cm or less in size, HR+/HER2-, and with no extensive ductal carcinoma in situ, invasive lobular carcinoma, or metastatic disease. The Michigan patients had one or more tumour characteristics outside of the ICE3 criteria, including tumour size greater than 1.5 cm, invasive lobular carcinoma and/or DCIS, tumours less than 0.5 cm from the skin, and ER/PR negative tumours.
Tumours, twice frozen
Cryoablation is based on the cytotoxic effects of cold that destroy cellular tissue, and consists of a first freeze, a passive thaw phase, and a second freeze. Cold temperatures freeze extracellular water, drawing water out of the cells and causing cellular dehydration. The cells swell and rupture during the passive thaw phase. The second freeze takes advantage of tissues that have been injured during the first freeze and conduct cold temperatures more efficient, enhancing the damaging effects of cold and expanding the area of tumour necrosis.
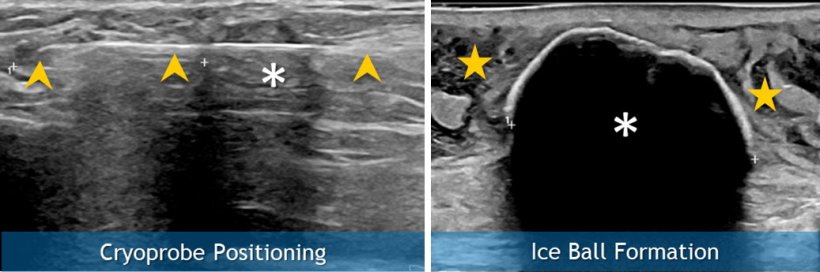
The procedure consists of using ultrasound imaging to guide a thin, needle-like device through the skin and into the breast tumour. The area is blasted with liquid nitrogen for 6 to 8 minutes at -40℃ or lower, followed by a 10-minute thaw, and a second 6- to 8-minute freeze.
Meeting patient requirements with ingenuity (and some paper tape)
Positioning of elderly patients can be difficult, especially for patients with large breasts, neck mobility issues, and the inability to extend an arm above their head
Sarah Moorman
‘We identified several challenges with our patients,’ said Moorman. ‘Superficial tumours risk skin necrosis during cryoablation. Because saline can dissipate rapidly in pendulous fatty breast tissue, we used a 21G needle for rapid saline infusion, had a dedicated operator managing saline needle positioning, and stocked the procedure room with extra bags of saline.’ She added: ‘Positioning of elderly patients can be difficult, especially for patients with large breasts, neck mobility issues, and the inability to extend an arm above their head. We recommend allowing extra time for the procedure, using a wedge for patient positioning and comfort, keeping the breast in position with paper tape, and placing the patient’s arm at their side or across the abdomen.’
‘Patients with comorbidities such as COPD or congestive heart failure may have shortness of breath and decreased oxygen, and risk hypoxia and fluid overload. We provide oxygen to these patients and elevate the head of the bed by as much as 45°. Targeting of the mass is likely feasible with needle entry from lateral, medial, or peripheral breast, but requires about 5-6 cm of tissue depth from surface,’ she explained.
Moorman advised that fat necrosis is expected to be seen on follow-up imaging, and that any changes near the cryoablation cavity suspicion findings should be promptly biopsied. All of the patients recovered rapidly, and none experienced serious side effects. There was one breast cancer recurrence, and one patient developed DCIS.
Profile:
Sarah E. H. Moorman, MD, is a breast imaging fellow at University of Michigan Health in Ann Arbor. Her interests include breast imaging and women’s imaging, with research interests including screening intervals and quality improvement.
29.11.2022