Article • Drug delivery
Biotherapeutics strike cancer cell growth
Many drug treatments do not work due to their poor ability to reach their intended targets inside patients’ cells. To address this, researchers at Cardiff University’s Schools of Pharmacy and Pharmaceutical Sciences, and Biosciences have designed a highly efficient method to improve the delivery of therapeutic molecules into diseased cells such as those in stomach cancer, breast cancer and tuberculosis.
Report: Mark Nicholls
The fact the same approach has worked for three very different receptors suggests that we should be looking at many different targets here, to do the same with them, spreading it away from cancer to other diseases.
Prof. Arwyn Jones
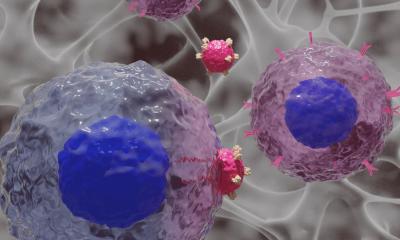
In this new approach, called ‘receptor crosslinking’, the team specifically worked to improve the delivery of a relatively new class of drugs called biotherapeutics. The researchers explain that cancer cells often contain a unique protein on their surface that acts as a barcode, identifying these cells as cancerous against their healthy counterparts.
In their findings, they characterised new ways of targeting breast cancer cells with Herceptin, which interacts specifically with a barcode protein called Her2 – a protein barcode widely recognised to be a major driver of cancer cell growth and division.
Lead author Professor Arwyn Jones, from the School of Pharmacy and Pharmaceutical Sciences, said: ‘The striking thing is that we have tested our approach on both Her2, as well as other barcode proteins, and each one gave the same result. ‘It looks like this could be a universal strategy to increase the uptake of drugs into different kinds of cells involved in many types of diseases.’
The research team has manipulated how Herceptin interacts with Her2, which results in both being rapidly engulfed by the cancer cells that then proceed to destroy the protein barcode.
Professor Jones: ‘The fact the same approach has worked for three very different receptors suggests that we should be looking at many different targets here, to do the same with them, spreading it away from cancer to other diseases.’
As well as different cancer types, the approach could be used to target inherited genetic diseases and infectious diseases such as tuberculosis. TB is a potential target, he explained, because it hides within macrophages (immune cells) and this delivery method might have potential to drag the drug to the inside of the macrophage ‘to hit the tuberculosis where it is hiding’.
Having highlighted the ‘universality of the approach’, the Jones’ team would now like to see other researchers try it with specific antibodies and models on which they are working. ‘Our approach is about more effective delivery of therapeutics to the inside of cells,’ he said. ‘If we think of cancer, many anti-cancer antibodies have now been developed to target receptors on the plasma membrane – some loaded with anti-cancer drugs.
‘The problem lies in then getting the antibody to the inside of cells, as its fate and that of its drug payload is governed by the targeted receptor.’
However, he added: ‘If you can force this interaction and have the receptor and antibody driven to the inside of a cell, you have a much better chance of getting that drug to its target site.’

Still, the expert acknowledges the approach is a long way from clinical use, despite its wide-reaching potential, but this a critical step forward. ‘The next stage,’ Professor Jones pointed out, ‘is to understand what happens inside a cell and then design drugs that specifically target that process.’
Profile:
Arwyn T Jones is Professor of Membrane Traffic and Drug Delivery at Cardiff University’s Schools of Pharmacy and Pharmaceutical Sciences. He is closely involved in the school’s main research themes of experimental therapeutics & pharmaceutical sciences; and drug discovery, design and synthesis. With a major interest in cancer and cell biology, his current projects fall under the overall themes of breast cancer cell biology, drug delivery and regulation of endocytosis. These include targeting and endocytosis of plasma membrane receptors, design and characterisation of drug delivery vectors and cellular delivery of therapeutic macromolecules.
22.07.2016