News • European Society for medical oncology
Androgen-deprivation treatments for prostate cancer could protect men from COVID-19
A study of 4532 men in the Veneto region of Italy has found that those who were being treated for prostate cancer with androgen-deprivation therapies (ADT) were less likely to develop the coronavirus COVID-19 and, if they were infected, the disease was less severe.
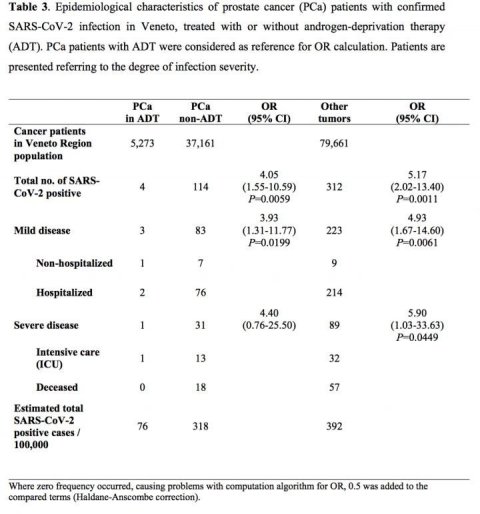
The authors of the study, say their findings suggest ADT appears to protect men from COVID-19 infection. The researchers, led by Professor Andrea Alimonti, from the Università della Svizzera Italiana (Bellinzona, Switzerland), found that out 4532 men infected with COVID-19, 9.5% (430) had cancer and 2.6% (118) had prostate cancer. Male cancer patients had a 1.8-fold increased risk of COVID-19 infection out of the whole male population and developed more severe disease.
However, when they looked at all prostate cancer patients in the Veneto region, they found that only four out of 5,273 men on ADT developed COVID-19 infection and none of them died. This compared to 37,161 men with prostate cancer who were not receiving ADT, of whom 114 developed COVID-19 and 18 died. Among 79,661 patients with other types of cancer, 312 developed COVID-19 and 57 died.
Prof Alimonti said: "Patients with prostate cancer receiving androgen-deprivation therapies had a significant four-fold reduced risk of COVID-19 infections compared to patients who did not receive ADT. An even greater difference was found when we compared prostate cancer patients receiving ADT to patients with any other type of cancer; there was a more than five-fold reduction in risk of infection among the prostate cancer patients on ADT.
"This is the first paper to suggest a link between ADT and COVID-19. We collected data from a large population of patients infected by the coronavirus and have found that those being treated with ADT for prostate cancer are protected, even though all patients with cancer have a greater risk of COVID-19 infection than non-cancer patients."
The researchers believe their findings suggest that even if men did not have prostate cancer, those who are at high risk of developing COVID-19 could take ADT for a limited period of time to prevent infection, while those who become infected could take ADT to reduce the severity of the symptoms.
"There are several clinically approved therapies that decrease the levels of androgens and that can be administered to patients. For instance, luteinizing hormone releasing hormone, or LH-RH, antagonists can decrease the levels of testosterone in patients in 48 hours and the effect of this therapy is transient. Once a patient stops taking the drug, his testosterone levels go back to the previous levels. These treatments to lower testosterone levels, if given for no more than a month, do not have major side effects," said Prof Alimonti.
"I hope that our findings inspire other clinicians to carry out clinical trials using transient ADT in men infected with COVID-19, in addition to other experimental therapies. Although these data need to be further validated in additional large cohorts of patients with COVID-19, they provide an answer to the hypothesis that androgen levels can facilitate coronavirus infections and increase the severity of symptoms, as has been seen in male patients."
Recommended article
News • Better diagnosis, better treatment
Prostate cancer deaths to decline (almost) everywhere in the EU
Death rates from prostate cancer are predicted to fall in 2020 in the EU, largely due to better diagnosis and treatment, according to new research published in the leading cancer journal Annals of Oncology. In the latest predictions for cancer deaths in the EU for 2020, researchers led by Carlo La Vecchia (MD), Professor at the School of Medicine, University of Milan (Italy), show that since 2015…
Prof Alimonti and his colleagues started to investigate the effect of ADT on vulnerability to COVID-19 this year after recent research showed that a protein called TMPRSS2 helped COVID-19 to infect healthy human cells. TMPRSS2 is a member of a family of proteins called Type II Transmembrane Serine Proteases, which are involved in a number of process in the body including cancer and viral infections. There are high levels of TMPRSS2 in prostate cancer patients and its action is regulated by the androgen receptor, at which therapies such as ADT is targeted. The androgen receptor also regulates TMPRSS2 levels in non-prostate tissues, including the lungs.
"This could explain why men infected by COVID-19 develop a more aggressive form of disease than women," said Prof Alimonti. "It is known that ADT can decrease the levels of TMPRSS2 in prostate cancer patients, and some experimental evidence demonstrates that this could happen not only in the prostate but also in other tissues. So I wanted to see if ADT could decrease the risk of developing coronavirus infection in men with prostate cancer."
The researchers suggest that androgen-deprivation therapies could be combined with other drugs that stop viruses multiplying and infecting human cells, or with drugs that interfere with the activity of TMPRSS2 in the body.
Limitations to the study include the fact that cancer patients with COVID-19 may have been tested for the virus more than non-cancer patients, since they are more often in hospital. This could explain the higher prevalence of coronavirus in cancer patients. Prostate cancer patients on ADT may be more careful about social distancing than those not on ADT and patients with other forms of cancer, especially as ADT can be administered at home.
Editor-in-chief of Annals of Oncology, Professor Fabrice André, Director of Research at the Institut Gustave Roussy, Villejuif, France, said: "We decided to publish this study because it provides a rationale to evaluate the efficacy of ADT prospectively in patients infected with COVID-19. Nevertheless, the study does not provide a definitive conclusion about the role of ADT in patients infected with COVID-19, and this class of drugs should not be used for this purpose until prospective trials have confirmed its efficacy."
Source: European Society for Medical Oncology
08.05.2020