Colorectal cancer screening
Aiming to raise safety and lower costs: The new NBI-based endoscopic classification (NICE)
Colon cancer remains the second most common cause of cancer-related death in the Western world with 450,000 citizens in Europe newly diagnosed and 230,000 deaths annually (Source: Globalscan 2008).

A rise to 90% in the five-year survival rate is seen if cancer is detected by endoscopic diagnosis and treated early enough.
Well known precursors for colon cancer are the adenomatous polyps that arise from the glandular structure of the colonic wall and differ from normal or hyperplastic tissue because they grow continuously and very likely develop dysplastic changes later on, eventually leading to invasive colonic cancer. The primary goal of any screening colonoscopy is therefore to detect and remove those adenomas.
Diagnosis and costs
Current clinical practice in colonoscopy is to detect and remove any polyp regardless whether harmless (hyperplastic) or adenomatous. The endoscopist is obliged to leave the final diagnosis of the polyp nature to a pathologist. Depending on number, size and sometimes with regards to national guidelines also depending on the details of the histopathology of the adenomas, the endoscopist will then define the surveillance interval: three or sometimes five or ten years if only diminutive hyperplastic lesions were detected. The economic downside of that well-established concept is the huge cost associated with the histopathological workup of retrieved polyps – particularly to evaluate diminutive polyps that are almost all benign anyway.
Given that over 14 million colonoscopies are performed annually in the USA, and assuming that 44% of them find at least one diminutive polyp, Kessler et al. (Endoscopy 2011; 43(8): 683-691, DOI: 10.1055/ s-0030-1256381) have estimated that the cost for evaluation exceed US$1 billion annually in the USA – ‘a conservative estimate that does not consider related costs such as the specimen container, pathologist time and endoscopist time to follow up on the pathology report,’ the authors state.
Cost-savings via optical diagnosis
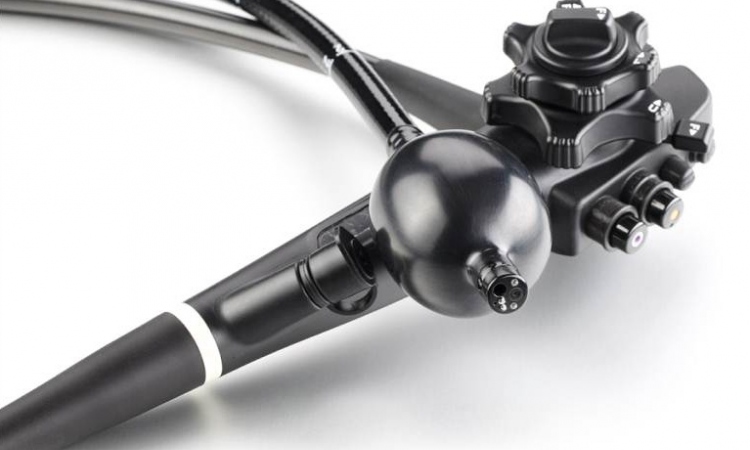
Recent advancements in endoscopic imaging such as Narrow Band Imaging (NBI) to improve visualisation of vessels, and High Definition (HD) imaging combined with dual focus for close up observation, encourage endoscopists worldwide in their attempts to change the state of care for diminutive polyps towards endoscopic optical diagnosis of the polyp nature, eventually allowing them to skip the costly pathologic analysis and discard all discovered and retrieved diminutive polyps.
Obviously such an approach can only work if a common, simple classification system for differentiation between adenomas and hyperplastic lesions is at hand. Therefore, recently an international group of renowned endoscopy experts suggested the so-called NBI International Colorectal Endoscopic (NICE) classification for assessment of small colorectal polyps (Hewett et al., Gastroenterology, Volume 143, Issue 3, September 2012, Pages 599- 607.) This classification is based on the observation of three major criteria using NBI in close-up view:
1. Colour: light vs. browner relative to background
2. Vessels: None, or isolated, lacy vessels coursing across the lesion vs. brown vessels surrounding white structures
3. Surface pattern: Dark or white spots of uniform size or homogeneous absence of pattern vs. oval, tubular or branched white structures surrounded by vessels. The former alternatives suggest a hyperplastic, the latter an adenomatous polyp.
Is optical diagnosis safe?
As stated previously, current guidelines do not allow endoscopists to completely rely on optical diagnosis of polyps. In the USA, to change the state of care the American Association of Endoscopists (ASGE) has issued a PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) (Gastrointestinal Endoscopy, Volume 73, No. 3: 2011) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. For colorectal polyps <=5 mm in size to be resected and discarded without pathologic assessment, endoscopic technology should provide at least 90% agreement in the assignment of post-polypectomy surveillance intervals when compared to decisions based on pathology assessment of all identified polyps. ‘The PIVI is intended to guide technology developers and clinical investigators toward the design and testing of technologies that address these important clinical needs in diminutive polyp management,’ the ASGE explains in the rationale for this PIVI: ‘Once endoscopic technologies meet an established PIVI threshold, those technologies are appropriate to be incorporated into clinical practice…’
What we need to make it happen
Some years ago, a first publication in the United Kingdom (Ignajatovic et. al. The Lancet Oncology, Volume 10, Issue 12, Pages 1171 - 1178, December 2009) already showed that optical diagnosis of small colorectal polyps during routine colonoscopy is feasible with 98% agreement between optical diagnosis and histopathology for screening intervals (British Guidelines) and 95% (US multi-society guidelines).
As for NICE, several studies are underway to collect further evidence that the classification can be safely used in a discard and resect regime using the latest hightech endoscopes (EVIS EXERA III, Olympus Europa Holding GmbH.).
However, providing clinical evidence is just the first step towards spreading the concept and eventually changing the state of care. Endoscopists will need extensive training on NICE, guidelines for colonoscopic examination and the histopathologic follow up will need revision, as will reimbursement schemes – changes such as those already established in Japan, where NBI-based optical diagnosis with zoom endoscopes already enjoys reimbursement.
Adequate collection and preservation of images is another issue to be covered and secured, particularly for medico-legal reasons.
PROFILE
Hepatogastroenterology expert and university professor Thierry Ponchon MD studied medicine at Lyon University and today heads the specialist department at the E. Herriot Hospital in Lyon, France. From 2001-2003 he served as President of SFED, the French Society of Digestive Endoscopy and has been President of the Society’s research and development commission since 1999. He also continues as the SFED’s International Secretary and is President of the organisation’s commission on hygiene and public health (since 2003). He has been Chairman of the Research Committee of the European Society of Digestive Endoscopy since 2001, of which he is also a member of the committees for education and guidelines. Prof. Ponchon is also a Member of the French National Society of Gastroenterology, and of the French Foundation of Digestive Oncology, where he is a member of the Scientific Committee. Given his standing in gastroenterology, endoscopy and lithotripsy, Prof. Ponchon additionally serves as a member of American, German, Brazilian and other international societies and scientific committees. Since 2000, he has been on the International Advisory Board of Digestive Endoscopy (Japan) and also has co-edited the journal Endoscopy since 2002. From 2004 he has also been a member of the International Editorial Board of Gastrointestinal Endoscopy (USA).
28.02.2013