Dividing T cells
Target for improving cancer immunotherapy
When an immune T cell divides into two daughter cells, the activity of an enzyme called mTORC1, which controls protein production, splits unevenly between the progeny, producing two cells with different properties. Such "asymmetric division," uncovered by Johns Hopkins Kimmel Cancer Center researchers using lab-grown cells and specially bred mice, could offer new ways to enhance cancer immunotherapy and may have other implications for studying how stem cells differentiate.
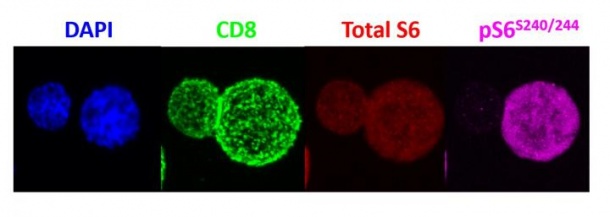
Results of their study, described in the June issue of Nature Immunology suggests that the uneven division of mTORC1 reprograms the daughter cells, so that one goes on to become an active immune system killer T cell, and the other becomes a memory T cell that persists, providing a constant source of antigen-specific T cells that can recognize threats like infections and cancer cells and reactivate the immune response against them.
Powell and his colleagues' experiments in mice showed that when a T "mother" cell that is naïve to immune threats encounters such a threat and divides, one of its daughter cells inherits far more mTORC1 activity compared with the other daughter cell. The difference in mTORC1 activity levels between the two daughter cells varied -- some were 2 to 3 times as much and some 10 times as much -- depending on the population of cells studied.
This lopsided distribution, he says, appears to reprogram the use of energy and other metabolic activities of each daughter cell so that the high-activity daughter goes on to generate the active immune system killers, called effector T cells, while the low-activity daughter generates memory T cells.
"One of the critical steps needed to improve cancer immunotherapy in general is finding out ways to make antitumor T cells persist or hang around in the body longer," says Jonathan Powell, M.D., Ph.D., a professor of oncology at the Johns Hopkins University School of Medicine and associate director of its Bloomberg-Kimmel Institute for Cancer Immunotherapy.
To determine the uneven distribution of mTORC1, the scientists activated mouse T cells with a specific immune-stimulating antigen (a virus in this case). Once activated, the T cells divided, and the scientists used antibodies to detect mTORC1 enzyme activity in each of the daughter cells. Then, Powell and his team sorted the two daughter cells and examined their function by injecting them into mice given two identical infections and tracked the cells' activity. The cells with high levels of mTORC1 activity were found to be potently activated, killer/effector T cells, while the cells with low mTORC1 levels behaved like memory T cells, persisting for long periods of time and rapidly activating upon reinfection.
One of the most significant aspects of the discovery, the researchers note, is the prospect that asymmetric partitioning of mTORC1 might be widespread across cells in many biological systems. Powell says it's possible that the mechanism may help explain how stem cells develop into more specialized cells in the bone marrow, for instance, or how cells differentiate from one another to become hair, skin, liver or brain cells in a growing embryo. "We think there will be implications for biology well beyond the immune system," says Powell.
Source: Johns Hopkins Medicine
04.07.2016