Image source: Waterhouse et al., Journal of Biomedical Optics 2023 (CC BY 4.0)
News • SWIR for intraoperative tumor delineation
Combining machine learning with multispectral infrared imaging to guide cancer surgery
The combination discerns healthy tissue from tumors based on their spectral profiles with higher accuracy than fluorescence-guided surgery alone
Surgical tumor removal remains one of the most common procedures during cancer treatment, with about 45 percent of cancer patients undergoing surgical tumor removal at some point. Thanks to recent progress in imaging and biochemical technologies, surgeons are now better able to tell tumors apart from healthy tissue. Specifically, this is enabled by a technique called “fluorescence-guided surgery” (FGS).
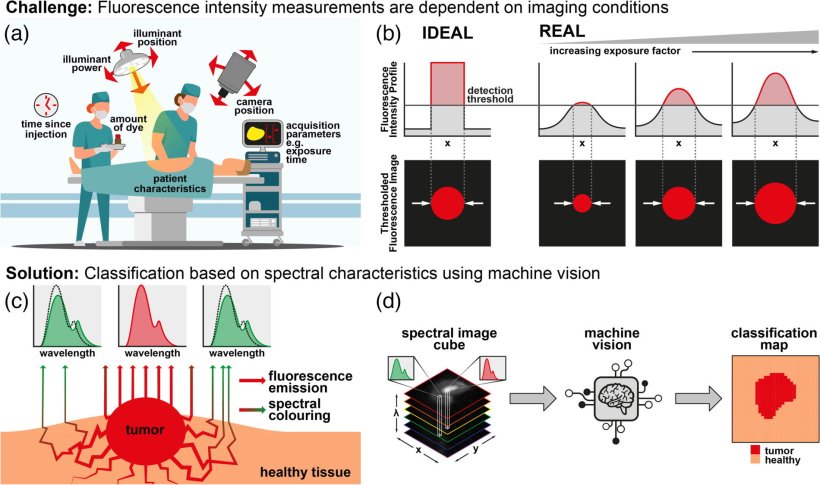
In FGS, the patient’s tissue is stained with a dye that emits infrared light when irradiated with a special light source. The dye preferentially binds to the surface of tumor cells, so that its lightwave emissions provide information on the location and extent of the tumor. In most FGS-based approaches, the absolute intensity of the infrared emissions is used as the main criterion for discerning the pixels corresponding to tumors. However, it turns out that the intensity is sensitive to lighting conditions, the camera setup, the amount of dye used, and the time elapsed after staining. As a result, the intensity-based classification is prone to erroneous interpretation.
But what if we could instead use an intensity-independent approach to classify healthy and tumor cells? A recent study published in the Journal of Biomedical Optics and led by Dale J. Waterhouse from University College London, UK, has now proposed such an approach. The research team has developed a new technique that combines machine learning with short-wave infrared (SWIR) fluorescence imaging to detect precise boundaries of tumors.
Recommended article
News • Fluorescence-guided surgery
"Lighting up" brain tumours for better surgical removal
A new real-time imaging technique that uses a type of infrared light has, for the first time, been used during surgery to differentiate between cancerous tumours and healthy tissue.
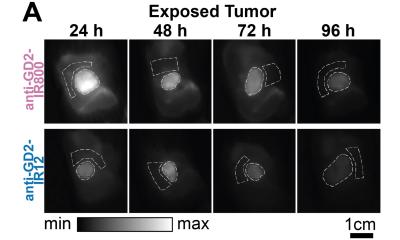
Their method relies on capturing multispectral SWIR images of the dyed tissue rather than simply measuring the total intensity over one particular wavelength. Put simply, the team sequentially placed six different wavelength frequency (color) filters in front of their SWIR optical system and registered six measurements for each pixel. This allowed the researchers to create the spectral profiles for each type of pixel (background, healthy, or tumor). Next, they trained seven machine learning models to identify these profiles accurately in multispectral SWIR images.
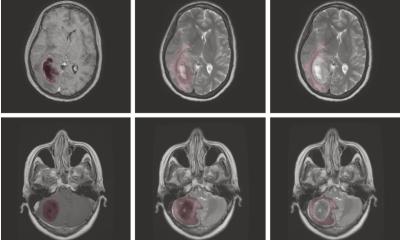
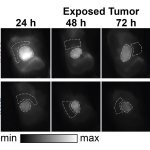
The researchers trained and validated the models in vivo, using SWIR images with a lab model for an aggressive type of neuroblastoma. They also compared different normalization approaches aimed at making the classification of pixels independent of the absolute intensity such that it was governed by the pixel’s spectral profile only.
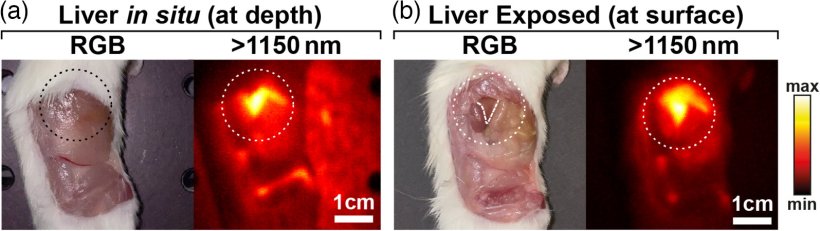
Image source: Waterhouse et al., Journal of Biomedical Optics 2023 (CC BY 4.0)
Out of the seven tested models, the best performing model achieved a remarkable per-pixel classification accuracy of 97.5% (the accuracies for tumor, healthy, and background pixels were 97.1%, 93.5%, and 99.2%, respectively). Moreover, thanks to the normalization of the spectral profiles, the results of the model were far more robust against changes in imaging conditions. This is a particularly desirable feature for clinical applications since the ideal conditions under which new imaging technologies are usually tested are not representative of the real-world clinical environment.
Based on their findings, the team has high hopes for the proposed methodology. They anticipate that a pilot study on its implementation in human patients could help revolutionize the field of FGS. Additionally, multispectral FGS could be extended beyond the scope of the present study. For example, it could be used to remove surgical or background lights from images, remove unwanted reflections, and provide noninvasive ways for measuring lipid content and oxygen saturation. Moreover, multispectral systems enable the use of multiple fluorescent dyes with different emission characteristics simultaneously, since the signals from each dye can be untangled from the total measurements based on their spectral profile. These multiple dyes can be used to target multiple aspects of disease, providing surgeons with even greater information.
Future studies will surely unlock the full potential of multispectral FGS, opening doors to more effective surgical procedures for treating cancer and other diseases.
Source: SPIE
30.03.2023