Image source: Shutterstock/Designua
Sponsored • Tools for the lab
Speeding up diagnostics to detect antibiotic resistance
Infectious disease diagnostics are notoriously slow. The gold standard for laboratory diagnosis of bacterial and fungal infection involves growing the pathogen from a clinical specimen – an overnight event, or even longer.
Author: Dr Jean Patel, Beckman Coulter

The healthcare focus is on improving the use of antibiotics for better patient outcomes and reducing the environmental pressures that drive antibiotic resistance. To impact antibiotic use, diagnostic tools need to be faster without compromising the performance characteristics necessary for definitive therapy decisions – a lot to ask, but this is a new era of innovation and companies are driven to fill this need.
Antimicrobial susceptibility test results enable definitive treatment decisions. This test traditionally takes three or more days from specimen collection to result, but technology is chipping away at the timeline. Molecular tests can identify the pathogen and detect resistance mechanisms from a positive blood culture in less than two hours. Knowing the infectious aetiology is powerful. This information alone can significantly reduce the time to de-escalate empiric therapy decisions to more targeted therapies.1
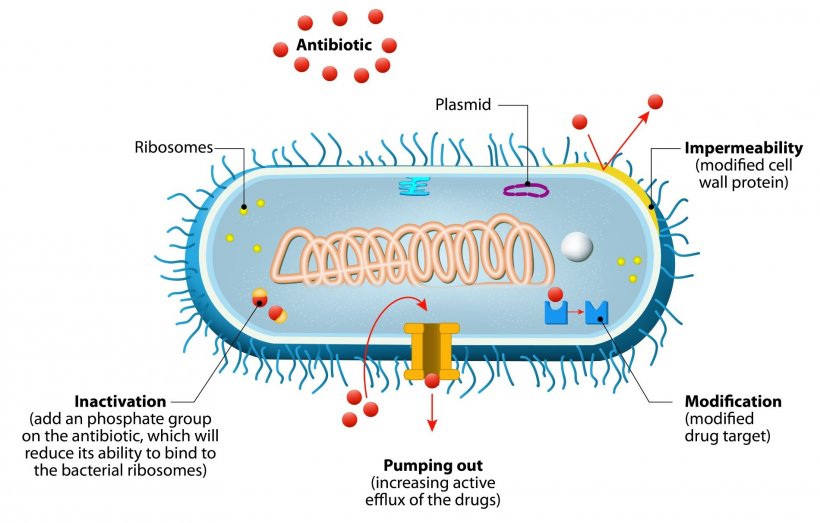
Detecting resistance mechanisms is most helpful for Gram-positive bacteria where a limited number of acquired resistance mechanisms are responsible for the most important phenotypes, like mecA in Staphylococcus aureus and van genes in Enterococcus. Assays to detect resistance mechanisms in Gram-negative bacteria can provide early evidence of what drugs will not work, but failure to detect a resistance mechanism is insufficient evidence to de-escalate antibiotics because many acquired resistance mechanisms and various chromosomal mutations can result in clinically significant phenotypic resistance. Sequence-based technologies can also identify pathogens, detect acquired resistance mechanisms, chromosomal mutations, and capture detailed isolate epidemiological features to map transmission dynamics (e.g., data to identify a common source of transmission).
Sequence-based assays are in development for clinical laboratories, but there are practical concerns like whether the price and complexity of testing are suitable for clinical laboratories. There is also a concern that molecular tests are limited to detection of known resistance and cannot detect resistance that is new or poorly characterised at the molecular level. With this limitation, how can molecular test results provide enough information to improve patient outcomes and antibiotic use?
Overnight phenotypic antimicrobial susceptibility testing is the gold standard test for definitive therapy decisions. Rapid AST systems have come to market, and more are in development. These aim to test positive blood cultures and the time to result ranges from two to six hours for the most common bacterial pathogens.
The following three examples are rapid AST systems that use very different technology.
- The Accelerate Pheno (www.acceleratediagnostics.com) systems will identify pathogens and perform rapid AST for the most common pathogens in positive blood culture and this is available in multiple markets. The method uses microscopic examination of cells in the presence of antibiotics as the detection method. The time to result for ID and AST is about seven hours. Over time, this system’s performance was improved to increase MIC accuracy and adding new drugs for reporting.2 The throughput is one specimen per instrument and the cost is greater than $100/specimen.
Detecting volatile organic chemicals from bacteria during growth
New to the European market is the Specific Diagnostics Reveal technology (www.specificdx.com). This test looks like a conventional MIC panel, but a lid is placed on the panel. This lid includes a small molecule sensor array to detect volatile organic chemicals produced by bacteria during growth. Changes in the array detection indicate growth or no growth in each well, thereby producing a MIC. The time to result is 4-6 hours and the stackable instrument can test two panels at a time. The Reveal assay is in development for the United States market.
- Flow cytometry, like the FastInov assay, is another detection method, which may be the fastest technology with results available in about two hours (www.fastinov.com). The throughput is lower because a single instrument queries each well of a microtiter plate.
A single specimen can take 30 or more minutes to read. However, with an overall time to result of two hours, this throughput may work well. This test is still in development.
These are just three examples. There is so much innovation in progress that one or more of these systems is sure to impact on medical practice significantly. Rapid phenotypic AST for positive blood culture is likely to become the standard of care. The winning tests will have an accuracy rate that can drive a definitive therapy solution in a single work shift and replace overnight AST. Where does the pathogen identification come from? The most likely answers are Maldi-TOF or molecular systems. Workflow and compatibility with rapid AST systems will determine each laboratory’s solution.
The next question worth answering is: Will rapid phenotypic AST replace all conventional AST? This would be an excellent win for patient care, but some technical breakthroughs are needed. Continuous innovation can get us to the goal.
References
1 (Banerjee R, Teng CB, Cunningham SA, et al. Randomised trial of rapid multiplex polymerase chain reaction-based blood culture identification and susceptibility testing. Clin Infect Dis 2015; 61:1071–80)
2 (Sikorski A, Shamsheyeva A, Gamage D, Oppermann N, Bhalodi AA, Humphries RM. 2021. Performance of antipseudomonal β-lactams on the Accelerate PhenoTest BC kit against a collection of Pseudomonas aeruginosa isolates. J Clin Microbiol 59:e01781-20)
Profile:
Dr Jean Patel joined Beckman Coulter after nearly seventeen years at the Center for Disease Control (CDC) in the Antimicrobial Resistance Reference Laboratory and the Office of Antimicrobial Resistance. Before this she was Assistant Professor of Pathology and Laboratory Medicine, and Assistant Director of Clinical Microbiology at the University of Pennsylvania.
16.09.2021